芘 | 129-00-0
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148 °C
-
沸点:393 °C
-
密度:1.271
-
闪点:210 °C
-
溶解度:可溶于乙醇
-
最大波长(λmax):330nm(EtOH)(lit.)
-
暴露限值:OSHA: TWA 0.2 mg/m3
-
LogP:5.43 at 30℃ and pH5-8
-
物理描述:Pyrene is a colorless solid, solid and solutions have a slight blue fluorescence. Used in biochemical research. (EPA, 1998)
-
颜色/状态:Monoclinic prismatic tablets from alcohol or by sublimation; pure pyrene is colorless
-
蒸汽压力:4.5X10-6 mm Hg at 25 °C
-
亨利常数:1.19e-05 atm-m3/mole
-
大气OH速率常数:5.00e-11 cm3/molecule*sec
-
稳定性/保质期:
-
本品有毒。急性中毒时,先引起兴奋,随后转为抑制、痉挛、四肢轻瘫,并伴有眼及上呼吸道黏膜刺激。实验数据显示,大鼠的LD₅₀值为0.17 mg/kg,小鼠的LD₅₀值为0.8 g/kg。操作人员应穿戴防护用具。
-
本品存在于香料烟烟叶和主流烟气中。
-
-
分解:Hazardous decomposition products formed under fire conditions - Carbon oxides.
-
燃烧热:3.878X10+7 J/kg at 25 °C
-
汽化热:3.21X10+5 J/kg
-
相对蒸发率:Evaporation at 20 °C is negligible
-
碰撞截面:136.1 Ų [M*]+; 137 Ų [M+H]+
-
保留指数:2069.7;2080.4;2126.4;2044;2095.8;2042;2061;2049.68;2060.85;2069.26;2069.4;2073.47;2091.45;2119;2101;2077;2085;2046;2068.4;2053.2;2061;346;348.1;350.3;351.2;351.8;347.41;348.12;348.91;350.67;350.72;351.42;351.74;353.42;352.6
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:16
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:T+,N
-
安全说明:S22,S28,S36/37,S45,S60,S61
-
危险类别码:R26,R36/37/38,R50/53
-
WGK Germany:2
-
海关编码:2902909090
-
危险品运输编号:UN 3077
-
危险类别:6.1(b)
-
RTECS号:UR2450000
-
包装等级:III
-
储存条件:贮存时应密封保存,并使用塑料袋包装。由于属于危险品,请按照危险品相关规定进行运输。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 芘;嵌二萘 |
| 化学品英文名称: | Pyrene |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 129-00-0 |
| 分子式: | C 16 H 10 |
| 分子量: | 202.26 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:芘;嵌二萘 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 未见急性中毒报道。长期接触3-5mg/m3,可见头痛、乏力、睡眠不佳、易兴奋、食欲减退、白细胞增加,血沉增速等。低于0.1mg/m3,未见不良影响。 |
| 环境危害: | |
| 燃爆危险: | 本品可燃。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用肥皂水及清水彻底冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。 |
| 食入: | 误服者给饮足量温水,催吐,就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。受高热分解放出有毒的气体。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、成分未知的黑色烟雾。 |
| 灭火方法及灭火剂: | 泡沫、二氧化碳、干粉、1211灭火剂、砂土。用水可引起沸溅。 |
| 消防员的个体防护: | 尽可能将容器从火场移至空旷处。 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 无资料 |
| 自燃温度(℃): | 引燃温度(℃):无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 切断火源。戴好防毒面具,穿化学防护服。收集运到空旷处焚烧。如大量泄漏,收集回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作。密闭操作,提供良好的自然通风条件。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防尘口罩,戴化学安全防护眼镜,穿防毒物渗透工作服,戴防化学品手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂接触。轻装轻卸。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:0.03mg/m3(皮) 美国TLV—TWA:未制 |
| 监测方法: | |
| 工程控制: | 密闭操作。提供良好的自然通风条件。 |
| 呼吸系统防护: | 一般不需特殊防护,但建议特殊情况下,佩带防毒面具。 |
| 眼睛防护: | 可采用安全面罩。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 必要时戴防化学品手套。 |
| 其他防护: | 工作后,淋浴更衣。避免长期反复接触。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色、棱形晶体(不纯物为黄色)。 |
| pH: | |
| 熔点(℃): | 150 |
| 沸点(℃): | 393.5 |
| 相对密度(水=1): | 1.27 |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 无资料 |
| 引燃温度(℃): | 引燃温度(℃):无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 16 H 10 |
| 分子量: | 202.26 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 不溶于水,溶于乙醇、乙醚等。 |
| 主要用途: | 用于制合成树脂、还原染料和分散性染料。直接氧化成芘醌,用于制还原染料。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、成分未知的黑色烟雾。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | 属低毒类 LD50:大鼠经口:2750mg/kg;小鼠经口:800mg/kg LC50:大鼠吸入:170mg/m3 |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | 家兔经皮: 500/24 小时,轻度刺激。 |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、干燥、通风良好的库房。远离火种、热源。保持容器密封。应与氧化剂分开存放。轻装轻卸。起运时包装要完整,装载应稳妥。运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂等混装混运。运输途中应防曝晒、雨淋,防高温。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 4 |
| MSDS修改日期: | 年月日 |
制备方法与用途
- 在高温焦油中含量约1.2%~1.8%,经分离、提纯制得芘。
- 以二蒽油为原料,通过真空精馏、萃取及重结晶工艺进行提纯。
- 使用蒸馏蒽油高于360℃的残渣作为原料,经过精馏切取芘馏分,并以苯为溶剂洗涤去盐基和不饱和化合物。再使用溶剂油进行重结晶,得到纯品芘。
芘又称嵌二萘,是煤焦油加工的产品之一,在煤焦油中的含量约为0.6~1.2%,主要集中在二蒽油馏分中。它是一种固体的芳香化合物,分子由四个苯环相连构成,其分子式为C₁₆H₁₀,分子量为202.26,熔点为150℃,沸点为393℃,密度为1.277g/cm³。芘呈淡黄色单斜片状结晶,不溶于水,易溶于苯、甲苯、二硫化碳及乙醚等有机溶剂。它是致癌物质,在二氧化氮的作用下会转化为能致突变的1-硝基芘。在与亲电试剂反应时,易发生在1位和6位,也可发生氧化、氢化等反应。此外,还可进行卤化、硝化和磺化等取代反应,一取代物为3位,二取代物则位于3,10-和3,8-位置,以3,10-位较多。当发生氧化时会生成3,10-芘醌及3,8-芘醌,进一步氧化可形成1,4,5,8-萘四甲酸。
提取方法:使用二蒽油馏分蒸馏过程中产生的渣油或沥青馏出物作为原料,在减压条件下进行精馏切取含芘约40%的390~400℃馏分。再用25%煤焦油溶剂油和75%乙醇混合溶剂(溶剂量与芘馏分之比为1:12)进行多次重结晶,直至产品合格。
化学反应有机化合物芘为淡黄色单斜晶体(纯品为无色),具有芳香性且可燃。不溶于水但易溶于乙醇、乙醚等有机溶剂。它可以进行亲电取代反应,如卤化、硝化和磺化等。
应用芘作为有机合成原料,经氧化后可用于制取1,4,5,8-萘四甲酸,进而用于生产染料、合成树脂、分散性染料及工程塑料。此外,通过酰化反应可以制造还原染料如艳橙GR及其他多种染料。也可用于制作杀虫剂和增塑剂。
化学性质芘为淡黄色单斜晶体,不溶于水但易溶于乙醚、二硫化碳、苯及甲苯中。利用重铬酸钾与乙酸或铬酸(H₂CrO₄)与醋酸氧化,可得芘-1,6-醒。亲电取代反应通常在1位发生,例如硝化、氯化和甲酰化等。
用途作为有机合成原料,经氧化可以制取1,4,5,8-萘四甲酸,并用于染料、合成树脂及工程塑料的生产;酰化后可制成还原染料艳橙GR及其他多种染料。还可以用于制造杀虫剂和增塑剂。
此外,直接氧化芘可得到1,4,5,8-萘四甲酸,用于染料、合成树脂、杀虫剂等产品的生产。作为合成树脂、还原染料和分散性染料的原料时,它可用于制备还原染料,并且可用作1,4,5,8-萘四甲酸生产的原料。
生产方法芘主要存在于煤焦油沥青的蒸馏物中。通过将中温沥青减压蒸馏,并向蒸馏釜通入少量直接过热蒸汽,切取窄馏分,再用溶剂油和乙醇混合溶液或苯与溶剂油的混合溶液进行重结晶,即可得到纯度达95%的工业芘。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 菲 phenanthrene 85-01-8 C14H10 178.233 萘 naphthalene 91-20-3 C10H8 128.174 —— azupyrene 193-85-1 C16H10 202.255 2-溴芘 2-bromopyrene 1714-27-8 C16H9Br 281.151 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— hexabenzo[a,c,fg,j,l,op]tetracene —— C40H22 502.615 —— dinaphtho[2,1,8,7-defg:2',1',8',7'-ijkl]pentaphene 188-90-9 C32H16 400.479 —— Dinaphtho<2,1,8,7defg;2',1',8',7'opqr>pentacene 188-91-0 C32H16 400.479 苯并[a]芘 3,4-benzopyrene 50-32-8 C20H12 252.315 苯并[e]芘 benzo[e]pyrene 192-97-2 C20H12 252.315 萘并(8,1,2-bcd)苝 naphtho[8,1,2-bcd]perylene 188-89-6 C26H14 326.397 二环戊烷并[Cd,Mn]芘 dicyclopenta[cd,mn]pyrene 96915-04-7 C20H10 250.299 —— cyclopent acepyrylene 98791-43-6 C20H10 250.299 —— dicyclopenta[cd,fg]pyrene 173678-72-3 C20H10 250.299 —— diindeno[1,2,3-cd:1',2',3'-jk]pyrene 191-23-1 C28H14 350.419 芘-2-酚 2-hydroxypyrene 78751-58-3 C16H10O 218.255 2-氯芘 2-Chlor-pyren 784-02-1 C16H9Cl 236.7 2-溴芘 2-bromopyrene 1714-27-8 C16H9Br 281.151 2,7-二溴芘 2,7-dibromopyrene 102587-98-4 C16H8Br2 360.048 2-氨基芘 2-aminopyrene 1732-23-6 C16H11N 217.27 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:(芳基)(氟芳基)钯配合物的还原消除机理研究:区域特异性脱氢交叉偶联的关键步骤摘要:交叉脱氢偶联(CDC)反应作为 C-C 键形成的短步合成方法而引起了人们的关注。最近,我们开发了萘和氟苯之间的CDC反应。该反应没有表现出一般的区域选择性,而是在萘的β位选择性地进行。在本研究中,使用模型配合物作为反应中间体的研究表明,独特选择性的起源是在β位上唯一发生还原消除。对还原消除的详细研究表明,萘基的空间位阻和氟苯的吸电子性质决定了还原消除反应进行的位置。这些结果表明,多环芳烃(PAH)的 C-H 官能化的选择性不是由 C-H 裂解步骤决定的,而是由随后的还原消除步骤决定。区域选择性 CDC 反应适用于各种 PAH,但对具有扩展 π 共轭的芘选择性较低。在氟苯底物中,C-H 部分两个邻位的 F 原子对于高选择性是必需的。底物范围与所提出的机制非常一致,其中还原消除步骤决定了区域选择性。DOI:10.1039/d4dt01453g
-
作为产物:描述:2-碘-1,3-二甲氧基苯 在 吡啶 、 bis-triphenylphosphine-palladium(II) chloride 、 tris(dibenzylideneacetone)dipalladium(0) chloroform complex 、 potassium fluoride 、 copper(l) iodide 、 [(t-BuXPhos)Au]NTf2 、 三溴化硼 、 四丁基碘化铵 、 potassium carbonate 、 三乙胺 、 tri tert-butylphosphoniumtetrafluoroborate 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 、 1,2-二氯乙烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 24.0h, 生成 芘参考文献:名称:Synthesis of Pyrenes by Twofold Hydroarylation of 2,6-Dialkynylbiphenyls摘要:阳离子金(I)络合物与Buchwald型二芳基膦有效催化了2,6-二炔基联苯的双重氢芳烃化反应,以构建芘骨架。DOI:10.1246/cl.2011.40
-
作为试剂:描述:参考文献:名称:利用电生成的高反应性锌方便地制备有机溴化锌及其在交叉偶联反应中的应用摘要:通过电解含有containing作为介体的DMF溶液以及铂阴极和锌阳极,可以轻松制备高反应性锌。发生refer的优先还原以生成相应的自由基阴离子,该自由基阴离子还原了由阳极溶解生成的锌离子,从而获得了具有高反应活性的零价锌。反应性锌已成功用于将溴代烷烃有效转化为相应的有机锌溴化物。所获得的有机锌溴化物进一步成功地用于Pd催化的与各种芳基碘化物和溴化物的交叉偶联反应中。DOI:10.1016/j.tet.2005.09.031
文献信息
-
White‐Fluorescent Dual‐Emission Mechanosensitive Membrane Probes that Function by Bending Rather than Twisting作者:Heorhii V. Humeniuk、Arnulf Rosspeintner、Giuseppe Licari、Vasyl Kilin、Luigi Bonacina、Eric Vauthey、Naomi Sakai、Stefan MatileDOI:10.1002/anie.201804662日期:2018.8.13Bent N,N′‐diphenyl‐dihydrodibenzo[a,c]phenazine amphiphiles are introduced as mechanosensitive membrane probes that operate by an unprecedented mechanism, namely, unbending in the excited state as opposed to the previously reported untwisting in the ground and twisting in the excited state. Their dual emission from bent or “closed” and planarized or “open” excited states is shown to discriminate between
-
一种芘酰亚胺衍生物及其合成方法和应用申请人:兰州大学公开号:CN108658993B公开(公告)日:2020-07-10
-
[EN] COMPOSITIONS AND METHODS FOR SYNTHESIS OF PHOSPHORYLATED MOLECULES<br/>[FR] COMPOSITIONS ET PROCÉDÉS DE SYNTHÈSE DE MOLÉCULES PHOSPHORYLÉES
-
MCR DENDRIMERS申请人:Wessjohann Ludger A.公开号:US20130203960A1公开(公告)日:2013-08-08The invention relates to a method for producing peptoidic, peptidic and chimeric peptidic-peptoidic dendrimers by multiple iterative multi-component reactions (MCR), in particular Ugi or Passerini multi-component reactions, to compounds produced in this way and to the use thereof.这项发明涉及一种通过多次迭代多组分反应(MCR),特别是Ugi或Passerini多组分反应,来制备肽样、肽和嵌合肽-肽样树状聚合物的方法,以及通过这种方式生产的化合物的用途。
-
Indolizine–Squaraines: NIR Fluorescent Materials with Molecularly Engineered Stokes Shifts作者:Louis E. McNamara、Tana A. Rill、Aron J. Huckaba、Vigneshraja Ganeshraj、Jacqueline Gayton、Rachael A. Nelson、Emily Anne Sharpe、Amala Dass、Nathan I. Hammer、Jared H. DelcampDOI:10.1002/chem.201702209日期:2017.9.12near infrared emissive materials with high quantum yields is an important challenge. Several classes of squaraine dyes have demonstrated high quantum yields, but require significantly red-shifted absorptions to access the NIR window. Additionally, squaraine dyes have typically shown narrow Stokes shifts, which limits their use in living biological imaging applications due to dye emission interference具有高量子产率的深红色和近红外发射材料的开发是一个重要的挑战。几类方酸菁染料已显示出高量子产率,但需要显着红移吸收才能进入NIR窗口。此外,方酸染料通常显示出狭窄的斯托克斯位移,这由于染料发射干扰光源而限制了它们在活体生物成像应用中的使用。通过结合吲哚并嗪杂环,我们合成了新颖的吲哚并嗪方酸染料,其斯托克斯位移增加(高达> 0.119 eV,增加了> 50 nm),并且比二氢吲哚方酸基准更高(N吸收最大726 nm和659 nm吸收最大)。 )。这些材料显示出显着增强的水溶性,对于不含水溶性取代基的方酸染料而言,这是独一无二的。进行了吸收,电化学,计算和荧光研究,观察到异常的荧光量子产率高达12%,发射曲线延伸超过850 nm。
表征谱图
-
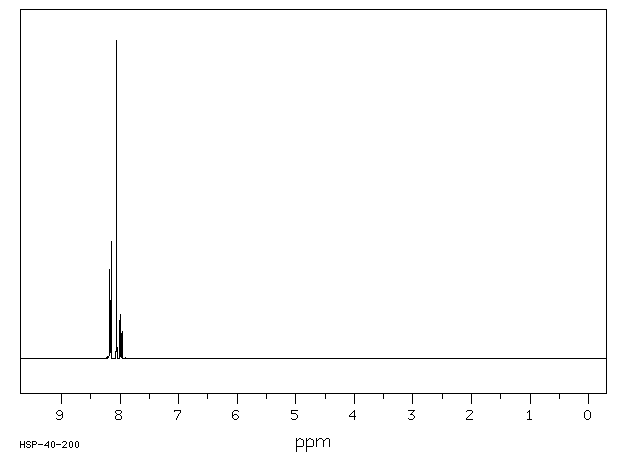
氢谱1HNMR
-
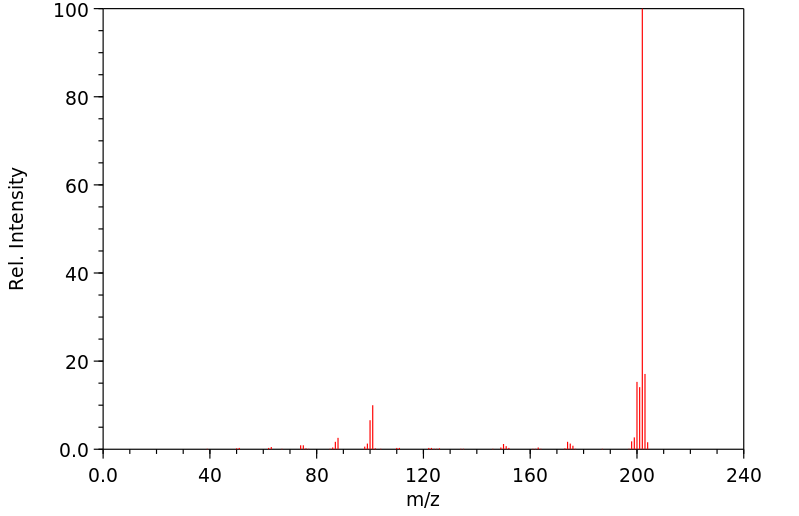
质谱MS
-
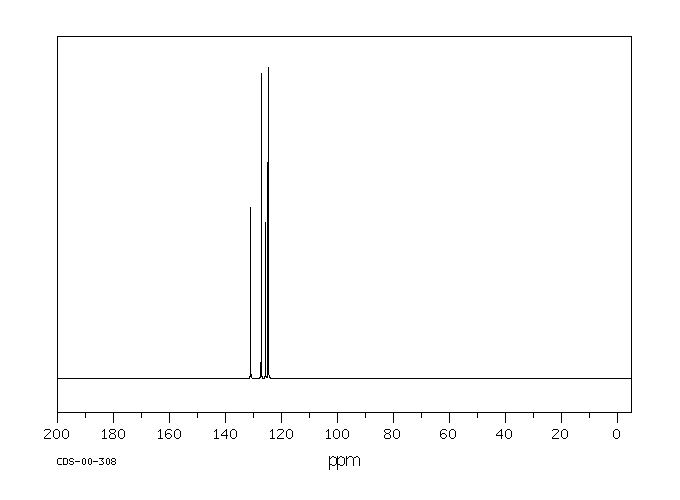
碳谱13CNMR
-
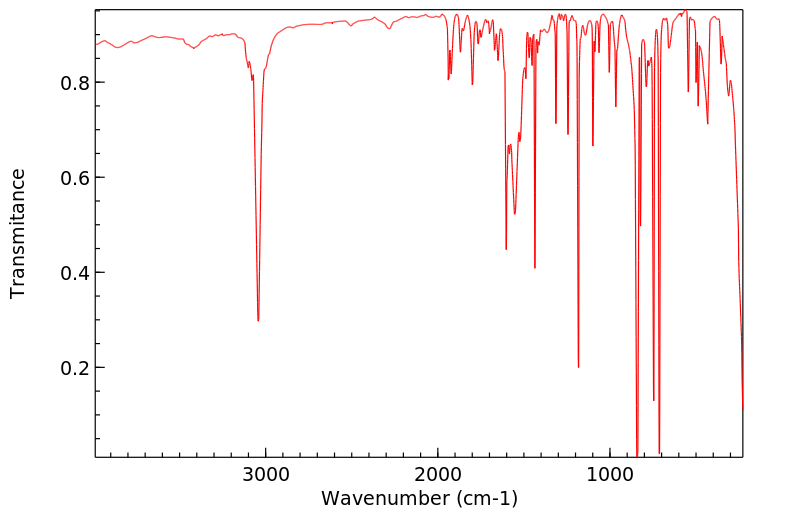
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息