萘 | 91-20-3
中文名称
萘
中文别名
萘丸;骈苯;并苯;煤焦油脑;粗萘;精萘
英文名称
naphthalene
英文别名
naphtalene
CAS
91-20-3
化学式
C10H8
mdl
MFCD00001742
分子量
128.174
InChiKey
UFWIBTONFRDIAS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80-82 °C (lit.)
-
沸点:218 °C (lit.)
-
密度:0.99
-
蒸气密度:4.4 (vs air)
-
闪点:174 °F
-
溶解度:可溶于甲醇:50mg/mL,澄清,无色
-
介电常数:2.5(85℃)
-
暴露限值:TLV-TWA 10 ppm (~50 mg/m3) (ACGIH, MSHA, and OSHA); STEL 15 ppm (~75 mg/m3) (ACGIH); IDLH 500 ppm.
-
LogP:3.7 at 25℃
-
物理描述:Naphthalene appears as a white crystalline volatile solid with a strong coal-tar odor. The solid is denser than water and insoluble in water. Burns, but may be difficult to ignite. . In the molten form it is very hot. Exposure to skin must be avoided. Also the vapors given off by the material may be toxic. Used as a moth repellent, fumigant, lubricants, and to make other chemicals, and for many other uses
-
颜色/状态:White crystalline flakes ... Plates from ethanol
-
气味:Aromatic odor
-
蒸汽密度:4.42 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.085 mm Hg at 25 °C
-
亨利常数:4.40e-04 atm-m3/mole
-
大气OH速率常数:2.16e-11 cm3/molecule*sec
-
稳定性/保质期:
-
使用五氧化二钒和硫酸钾作催化剂,硅胶作为载体,在385-390℃条件下用空气氧化得到邻苯二甲酸酐。在乙酸溶液中使用氧化铬进行氧化可生成α-萘醌,进一步加氢则产生四氢化萘,继续加氢可得十氢化萘。以氯化铁为催化剂,将氯气通入萘的苯溶液中,主要产物是α-氯萘。在光照条件下与氯作用,则主要生成四氯化萘。萘的硝化比苯更易进行,在常温下即可进行,主要产物为α-硝基萘。萘的磺化产物受温度影响,低温下得到α-萘磺酸,而在较高温度时则主要产生β-萘磺酸。
-
萘具有较小的水溶性且不易被人体吸收,因此其毒性较弱。吸入高浓度的萘蒸气或萘粉末可能导致呕吐、不适和头痛,特别会损害眼角膜,引起小水泡及点状浑浊,并可能引发皮肤炎症,有时还会影响肺部健康,造成病理改变;此外,长期接触还可能对肾脏产生影响,导致血尿。尽管如此,萘并没有致癌性。根据相关规定,在工作场所中萘的最大允许浓度为10×10-6。为了安全起见,生产设备及容器应保持密闭状态,防止蒸气或粉末逸出,并在操作现场强制进行通风。若发生中毒症状,则需立即将患者移至新鲜空气中,并让其多饮水、催吐;如有严重情况,请立即送医治疗。
-
稳定性:萘较为稳定。
-
不会发生聚合反应。
-
-
自燃温度:979 °F (526 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
粘度:0.754 cP at 20 °C
-
腐蚀性:Melted naphthalene will attack some forms of plastics, rubber, and coatings.
-
燃烧热:-16,720 BTU/lb = -9287 cal/g = -388.8X10+5 Joules/kg
-
汽化热:352 kJ/kg
-
表面张力:Liquid surface tension: 31.8 dynes/cm = 0.0318 Newtons/m at 100 °C
-
电离电位:8.12 eV
-
气味阈值:Odor detection in water 6.80 ppm (purity not specified)
-
折光率:Index of refraction: 1.4003 at 24 °C/D
-
相对蒸发率:Much less than 1. (Butyl acetate= 1)
-
碰撞截面:120.28 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
-
保留指数:200
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
经实验表明,24-35%的腹腔注射剂量(14)C-萘在大鼠和小鼠体内以巯基尿酸形式在24小时内排出。对于这两种动物,这一百分比在较宽的剂量范围内(3.12-200 mg/kg体重)保持一致。相比之下,在吸入暴露后,小鼠尿液中巯基尿酸的量大约是同等暴露水平下大鼠尿液中的两倍。在24小时内,给予小鼠50-200 mg/kg体重萘的腹腔注射,大约有100-500 umol/kg体重的巯基尿酸通过尿液排出。而小鼠在吸入1-100 ppm(5.24-524 mg/m³)的萘4小时后,通过尿液排出的总巯基尿酸量为1-240 umol/kg体重,而暴露于相同浓度的大鼠排出的量为0.6-67 umol/kg体重。
/It was shown/ that 24-35% of an intraperitoneal dose of (14)C-naphthalene was eliminated as mercapturates by both mice and rats at 24 hours after dosing. For both species, this percentage was the same over a wide dose range (3.12-200 mg/kg body weight). In contrast, after inhalation exposure, the amounts of mercapturic acid in mouse urine were approximately twice those in rat urine at the same level of exposure. Over a 24 hour period, approximately 100-500 umol/kg body weight mercapturates were eliminated in urine of mice given intraperitoneal injections of 50-200 mg/kg body weight naphthalene. In mice exposed by inhalation to 1-100 ppm (5.24-524 mg/cu m) naphthalene for 4 hours, 1-240 umol/kg body weight total mercapturic acids were eliminated, while rats exposed to the same concentrations eliminated 0.6-67 umol/kg body weight.
来源:Hazardous Substances Data Bank (HSDB)
代谢
一只5天大的小牛口服了标记有14C的毒死蜱,70%的剂量以胱氨酸结合物的形式通过尿液排出;没有检测到巯基尿酸。在小牛(5周大)中建立了瘤胃微生物群,并重复了相同的实验,得到了相同的结果。当同一只小牛在一周后口服了标记有14C的萘,99%的剂量通过尿液排出,大部分是作为二氢二醇葡萄糖苷酸(34%)和二氢羟基胱氨酸结合物(47%);没有检测到巯基尿酸。...小牛肾脏和肝脏中的胱氨酸S-结合物N-乙酰转移酶活性大约是对应大鼠组织的10%。
A 5 day old calf dosed orally with (14)C-propachlor excreted 70% dose in the urine as the cysteine conjugate; no mercapturic acid was detected. Rumen microflora were established in the calf (5 weeks older) and the experiment was repeated with the same results. When the same calf was dosed 1 week later with (14)C-naphthalene, 99% dose was excreted in the urine, mostly as the dihydrodiol glucuronide (34%) and the dihydrohydroxy cysteine conjugate (47%); no mercapturate was detected. ... Cysteine S-conjugate N-acetyltransferase activity in calf kidney and liver was about 10% of that n the corresponding rat tissues.
来源:Hazardous Substances Data Bank (HSDB)
代谢
通过1,2-环氧化反应转化为1,2-二氢萘-1,2-二醇、1,2-二氢-1-萘酚和N-乙酰-S-(2-羟基-1,2-二氢萘基)-半胱氨酸,这些物质在进一步代谢后...以1-萘基硫酸酐的形式从尿液中排出...以及1,2-二氢萘-1,2-二醇的共轭物...1-和2-萘酚以及1,2-二羟基萘。
...Metabolized via 1,2-epoxide into 1,2-dihydronaphthalene-1,2-diol, 1,2-dihydro-1-naphthol and N-acetyl-s-(2-hydroxy-1,2-dihydronaphthyl)-cysteine, which after further metabolism... Excreted in urine as 1-naphthylmercapturic acid ...and conjugates of 1,2-dihydronaphthalene-1,2-diol... 1- and 2-naphthols, and 1,2-dihydroxynaphthalene.
来源:Hazardous Substances Data Bank (HSDB)
代谢
萘首先被代谢成萘1,2-氧化物,这可以产生1-萘酚或者通过环氧水解酶转化为反式-1,2-二氢-1,2-羟基萘(反式-1,2-二氢二醇)。1-萘酚的羟基也可以被硫酸化或葡萄糖酸化。1,2-二氢二醇还可以转化为2-萘酚。环氧也是谷胱甘肽S-转移酶的底物,产生谷胱甘肽结合物,最终以巯基尿酸的形式排出体外。
Naphthalene is metabolized first to naphthalene 1,2-oxide, which can yield 1-naphthol or be converted by epoxide hydrolase to trans-1,2-dihydro- 1,2-dihydroxynaphthalene (trans-1,2-dihydrodiol . The hydroxyl group of 1-naphthol may also be sulfated or glucuronidated. The 1,2-dihydrodiol can also be converted to 2-naphthol. The epoxide is also a substrate for glutathione S-transferase, yielding glutathione conjugates which are eventually eliminated as mercapturic acids.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Naphthalene has known human metabolites that include 1-Naphthol, 2-Naphthol, naphthalene-1,2 Expodation, and dihydrodiol.
来源:NORMAN Suspect List Exchange
毒理性
多环芳烃(PAHs)如萘在绑定血液蛋白如白蛋白后会在体内传输。与芳基烃受体或甘氨酸N-甲基转移酶结合会诱导细胞色素P450酶(尤其是CYP1A1、CYP1A2和CYP1B1)的表达。这些细胞色素酶将多环芳烃代谢成各种有毒中间体(环氧化物中间体、二氢二醇、酚、醌及其各种组合)。多环芳烃的活性代谢物与DNA和其他细胞大分子共价结合,引发突变和致癌作用(10、12、2、3)。在人类中,代谢物α-萘酚与一些病例中摄入或大量皮肤或吸入暴露后发展成溶血性贫血有关。易感性似乎会因为葡萄糖-6-磷酸脱氢酶(G-6-PD)的缺乏而加剧。超过4亿人患有称为葡萄糖-6-磷酸脱氢酶缺乏的遗传病。接触萘对这些人的危害更大,可能在较低剂量下就引起溶血性贫血。一些萘代谢物会耗尽受影响组织(如肺部)中的谷胱甘肽储备,导致毒性。负责耗尽谷胱甘肽的代谢物已被识别为萘氧化物或1,2-萘醌和1,4-萘醌。
PAH's such as naphthalene are transported throughout the body after binding blood proteins such as albumin. Binding to the aryl hydrocarbon receptor or glycine N-methyltransferase induces the expression of cytochrome P450 enzymes (especially CYP1A1, CYP1A2, and CYP1B1). These cytochrome enzymes metabolize PAH's into various toxic intermediates (epoxide intermediates, dihydrodiols, phenols, quinones, and their various combinations). The reactive metabolites of PAHs covalently bind to DNA and other cellular macromolecules, initiating mutagenesis and carcinogenesis. (10, 12, 2, 3). In humans, the metabolite alpha-naphthol has been linked to the development of hemolytic anemia in some cases following ingestion or extensive dermal or inhalation exposure. Susceptibility appears to be exacerbated by a deficiency in the glucose 6-phosphate dehydrogenase enzyme, or G-6-PD. Over 400 million people have an inherited condition called glucose-6-phosphate dehydrogenase deficiency. Exposure to naphthalene is more harmful for these people and may cause hemolytic anemia at lower doses. Some naphthalene metabolites deplete glutathione stores in affected tissues such as the lungs, leading to toxicity. The metabolites responsible for glutathione depletion have been identified as naphthalene oxide or 1,2-naphthoquinone and 1,4-naphthoquinone.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
证据权重特征:根据1986年致癌风险评估指南的标准,萘被分类为C组,即可能的 human致癌物。这是基于对通过口服和吸入途径暴露于萘的人类致癌性数据不足,以及通过吸入途径对动物致癌性的有限证据。使用1996年提出的致癌风险评估指南,目前基于人类和动物数据,无法确定萘通过口服或吸入途径的人类致癌潜力;然而,有暗示性证据(观察到只有雌性小鼠通过吸入萘暴露出现了良性呼吸道肿瘤和一个 carcinoma)。额外的支持包括与暴露于1-甲基萘相关的呼吸道肿瘤增加。目前,萘产生良性呼吸道肿瘤的机制尚未完全了解,但假设涉及通过细胞色素P-450单加氧酶系统产生的氧化反应代谢物。然而,基于在遗传毒性测试中获得的许多阴性结果,遗传毒性机制似乎不太可能。人类致癌性数据:现有数据不足以建立暴露于萘与人类癌症之间的因果关系。没有找到充分规模的流行病学研究,旨在检查萘暴露与癌症之间的可能关联。总体而言,没有可用于评估暴露人类群体致癌潜力的数据。
WEIGHT-OF-EVIDENCE CHARACTERIZATION: Using criteria of the 1986 Guidelines for Carcinogen Risk Assessment, naphthalene is classified in group C, a possible human carcinogen. This is based on the inadequate data of carcinogenicity in humans exposed to naphthalene via the oral and inhalation routes, and the limited evidence of carcinogenicity in animals via the inhalation route. Using the 1996 Proposed Guidelines for Carcinogen Risk Assessment, the human carcinogenic potential of naphthalene via the oral or inhalation routes "cannot be determined" at this time based on human and animal data; however, there is suggestive evidence (observations of benign respiratory tumors and one carcinoma in female mice only exposed to naphthalene by inhalation). Additional support includes increase in respiratory tumors associated with exposure to 1-methylnaphthalene. At the present time the mechanism whereby naphthalene produces benign respiratory tract tumors are not fully understood, but are hypothesized to involve oxygenated reactive metabolites produced via the cytochrome P-450 monooxygenase system. However, based on the many negative results obtained in genotoxicity tests, a genotoxic mechanism appears unlikely. HUMAN CARCINOGENICITY DATA: Available data are inadequate to establish a causal association between exposure to naphthalene and cancer in humans. Adequately scaled epidemiological studies designed to examine a possible association between naphthalene exposure and cancer were not located. Overall, no data are available to evaluate the carcinogenic potential in exposed human populations.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A3:已确认的动物致癌物,对人类的相关性未知。
A3: Confirmed animal carcinogen with unknown relevance to humans.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Evaluation: There is inadequate evidence in humans for the carcinogenicity of naphthalene. There is sufficient evidence in experimental animals for the carcinogenicity of naphthalene. Overall evaluation: Naphthalene is possibly carcinogenic to humans (Group 2B).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
萘基于在实验动物研究中的充分证据,合理预期对人是一种致癌物。
Naphthalene is reasonably anticipated to be a human carcinogen based on sufficient evidence from studies in experimental animals.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
婴儿皮肤对萘的吸收会因婴儿油而增加。
Cutaneous absorption of naphthalene in infants is increased by baby oil.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
吸入时,萘迅速被吸收……
When inhaled, naphthalene is rapidly absorbed... .
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在100毫克/公斤腹膜内给药时,在大鼠尿液中排出了20至30%,并且85至90%的尿液排出物是酸结合物形式;5至10%通过胆汁排出,70至80%的胆汁排出物也是酸结合物。主要的代谢物是萘-1,2-二氢二醇。
At 100 mg/kg intraperitoneally, 20 to 30% was excreted in the rat urine, and 85 to 90% /of urinary excretion/ was in the form of acid conjugates; 5 to 10% was excreted in the bile and 70 to 80% /of biliary excretion/ was as acid conjugates. The major metabolite was naphthalene-1,2-dihydrodiol.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
In small oysters transport of naphthalene between tissues is primarily by diffusion. In intact oysters, accumulation in adductor muscle and body followed accumulation in gills after a large lag-time. In isolated tissues with no shell to impede water, there was no time lag.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 10 ppm (50 mg/m3), STEL: 15 ppm (75 mg/m3)
-
TSCA:Yes
-
危险等级:4.1
-
立即威胁生命和健康浓度:250 ppm
-
危险品标志:Xn,N
-
安全说明:S16,S36/37,S45,S46,S60,S61,S62,S7
-
危险类别码:R22,R40,R50/53
-
WGK Germany:3
-
海关编码:2707400000
-
危险品运输编号:UN 1334 4.1/PG 3
-
危险类别:4.1
-
RTECS号:QJ0525000
-
包装等级:III
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,库温不宜超过35℃。 - 包装密封,并与氧化剂分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有合适的材料收容泄漏物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 蒽 anthracene 120-12-7 C14H10 178.233 菲 phenanthrene 85-01-8 C14H10 178.233 苯并[a]菲 chrysene 218-01-9 C18H12 228.293 7H-苯并环庚烯-7-酮 benzocyclohepten-7-one 4443-91-8 C11H8O 156.184 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 蒽 anthracene 120-12-7 C14H10 178.233 并四苯 Tetracen 92-24-0 C18H12 228.293 苯并[a]菲 chrysene 218-01-9 C18H12 228.293 4-(5-(9,9-二辛基-9H-芴-2-基)-4-己基噻吩-2-基)-7-(4-己基噻吩-2-基)苯并[c][1,2,5]噻二唑 terrylene 188-72-7 C30H16 376.457 苯并[G]屈 benzo[g]chrycene 196-78-1 C22H14 278.353 菲 phenanthrene 85-01-8 C14H10 178.233 苉 picene 213-46-7 C22H14 278.353 苝 PERYLENE 198-55-0 C20H12 252.315 苯并[a]蒽 benz[a]anthracene 56-55-3 C18H12 228.293 芘 pyrene 129-00-0 C16H10 202.255 苯并-3,4-菲 benzo[c]phenathrene 195-19-7 C18H12 228.293 9,10-苯并菲 triphenylene 217-59-4 C18H12 228.293 苯并[e]芘 benzo[e]pyrene 192-97-2 C20H12 252.315 苯并[a]芘 3,4-benzopyrene 50-32-8 C20H12 252.315 足球烯 fullerene C60 99685-96-8 C60 720.66 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Fuson, Journal of the American Chemical Society, 1926, vol. 48, p. 1096摘要:DOI:
-
作为产物:参考文献:名称:Dealkylation of alkyl naphthalenes in the presence of organo-aluminum catalyst摘要:公开号:US02880251A1
-
作为试剂:描述:在 噻吩-2-甲酸亚铜(I) 、 萘 、 C57H46N2O2 、 sodium 、 potassium carbonate 、 四(3,5-二(三氟甲基)苯基)硼酸钠 作用下, 以 四氢呋喃 、 邻二甲苯 、 丙酮 、 甲苯 为溶剂, 反应 120.02h, 生成参考文献:名称:铜催化通过 α-亚氨基铜卡宾对叠氮酰胺进行对映选择性去对称 C(sp2)–H 官能化摘要:α-亚氨基金属卡宾是有机合成中的多功能中间体,在不同的N-杂环的组装中具有广泛的应用。然而,基于α-亚氨基金属卡宾的催化对映选择性去对称化迄今为止尚未开发出来。在此,我们公开了通过α-亚氨基铜卡宾对叠氮-炔酰胺进行对映选择性去对称化C(sp 2 )–H官能化,从而以中等至优异的产率和高对映选择性有效组装不同的手性吲哚氮杂卓。值得注意的是,该反应代表了第一个基于 α-亚氨基金属卡宾的对映选择性去对称反应。进一步的合成转化和生物测试显示了该方法的潜在效用。此外,还采用计算研究来阐明反应机理和对映选择性的起源。DOI:10.1007/s11426-024-1990-y
文献信息
-
A Method for the Net Contra-thermodynamic Isomerization of Cyclic Trisubstituted Alkenes作者:Raphaël F. Guignard、Laurent Petit、Samir Z. ZardDOI:10.1021/ol4018744日期:2013.8.16A simple sequence for the net contra-thermodynamic isomerization of cyclic trisubstituted alkenes is reported consisting of a radical addition of p-chlorothiophenol, followed by oxidation to the sulfoxide and thermal syn-elimination to give the least substituted isomeric cycloalkene.为环状三取代烯烃的净禁忌热力学异构化的简单序列报道由自由基加成的p -chlorothiophenol,随后氧化成亚砜和热合成β-消除,得到至少取代的同分异构的环烯烃。
-
Nickel-catalyzed reductive defunctionalization of esters in the absence of an external reductant: activation of C–O bonds作者:Yasuaki Iyori、Kenjiro Takahashi、Ken Yamazaki、Yusuke Ano、Naoto ChataniDOI:10.1039/c9cc07710c日期:——
The nickel-catalyzed reductive cleavage of esters in the absence of an external reductant, which involves the cleavage of an inert acyl C–O bond in
O -alkyl esters is reported.镍催化剂在无外部还原剂的情况下,报告了酯的还原裂解,其中涉及对惰性酰基C-O键进行裂解,这种裂解发生在O-烷基酯中。 -
Copper-Catalyzed Protodecarboxylation of Aromatic Carboxylic Acids作者:Lukas J. Gooßen、Werner R. Thiel、Nuria Rodríguez、Christophe Linder、Bettina MelzerDOI:10.1002/adsc.200700223日期:2007.10.8A catalyst generated from copper(I) oxide and 4,7-diphenyl-1,10-phenanthroline for the first time allows the catalytic protodecarboxylation even of deactivated aromatic carboxylic acids, giving rise to the corresponding arenes. Based on DFT calculations, a reaction pathway is proposed that accurately reflects the experimental results, such as the observed reactivity order of the substrates.
-
Hydrazines and Azides via the Metal-Catalyzed Hydrohydrazination and Hydroazidation of Olefins作者:Jérôme Waser、Boris Gaspar、Hisanori Nambu、Erick M. CarreiraDOI:10.1021/ja062355+日期:2006.9.1which the H and the N atoms come from two different reagents, a silane and an oxidizing nitrogen source (azodicarboxylate or sulfonyl azide). The hydrohydrazination reaction using di-tert-butyl azodicarboxylate is characterized by its ease of use, large functional group tolerance, and broad scope, including mono-, di-, tri-, and tetrasubstituted olefins. Key to the development of the hydroazidation报道了 Co 和 Mn 催化的烯烃加氢肼和加氢叠氮化反应的发现、研究和实施。这些反应等效于 CC 双键与受保护的肼或偶氮酸的直接加氢胺化,但基于不同的概念,其中 H 和 N 原子来自两种不同的试剂,硅烷和氧化性氮源(偶氮二羧酸或磺酰叠氮化物) )。使用偶氮二羧酸二叔丁酯的加氢肼反应具有使用方便、官能团耐受性大、适用范围广的特点,包括单、二、三和四取代烯烃。氢叠氮化反应发展的关键是使用磺酰叠氮化物作为氮源和叔丁基过氧化氢的活化作用。发现该反应对于单、二和三取代烯烃的官能化是有效的,并且只有少数官能团是不能容忍的。获得的烷基叠氮化物是通用中间体,可以在不分离叠氮化物的情况下转化为游离胺或三唑。初步的机理研究表明,烯烃的氢化钴是限速的,然后是胺化反应。不能排除并可能涉及自由基中间体。然后进行胺化反应。不能排除并可能涉及自由基中间体。然后进行胺化反应。不能排除并可能涉及自由基中间体。
-
Regiospecific Cleavage of S–N Bonds in Sulfonyl Azides: Sulfonyl Donors作者:Zhiguo Zhang、Songnan Wang、Yong Zhang、Guisheng ZhangDOI:10.1021/acs.joc.8b03046日期:2019.4.5Sulfonyl azides have been widely used as sulfonamido, diazo, and azido donors, as well as all-nitrogen 1,3-dipoles donors in synthetic chemistry. Here, the sulfonyl azides were used as efficient sulfonyl donors, which is very unusual. Trifluoromethanesulfonic acid-induced formation of the sulfonyl cation reactive species from sulfonyl azides was developed and used for the first time to couple various
表征谱图
-
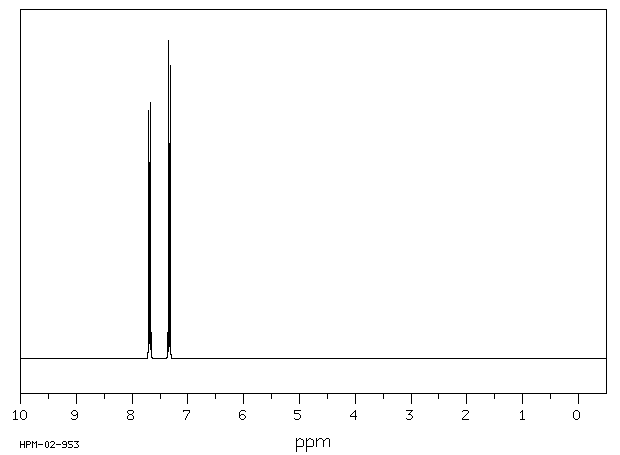
氢谱1HNMR
-
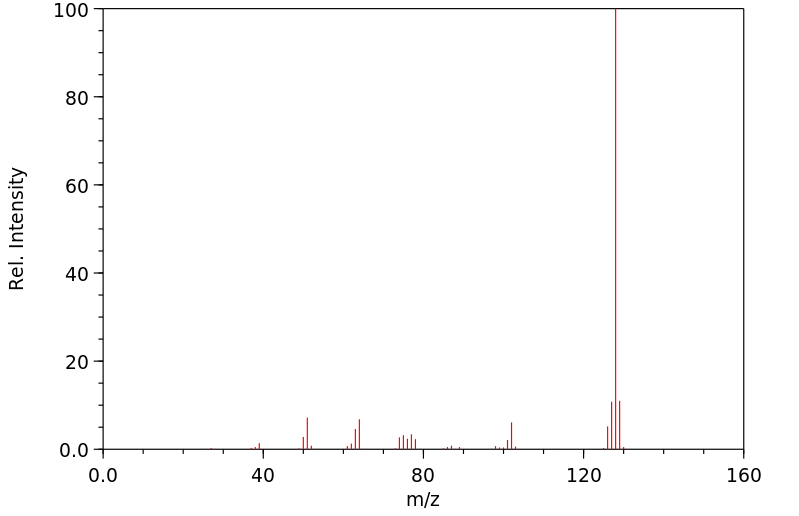
质谱MS
-
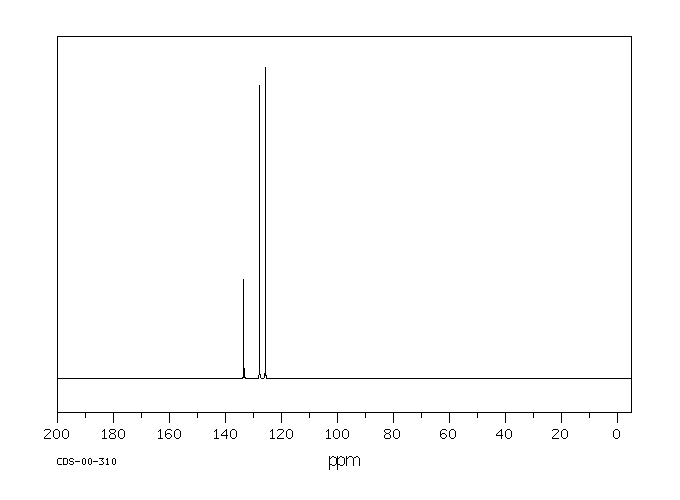
碳谱13CNMR
-
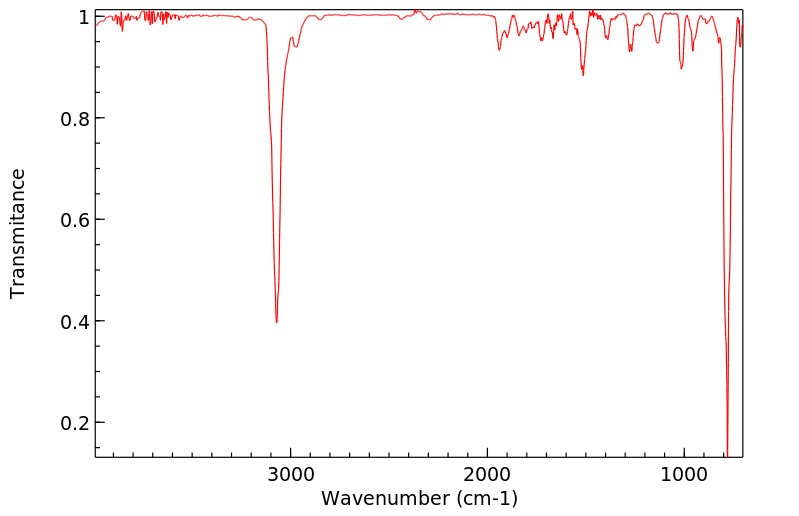
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮