二乙二醇乙醚 | 111-90-0
物质功能分类
中文名称
二乙二醇乙醚
中文别名
乙氧基二甘醇;乙基卡必醇;二甘醇单乙醚;二乙二醇单乙醚;二甘醇一乙醚;二乙二醇单乙基醚;二甘醇乙醚;二乙二醇独乙醚;二乙二醇一乙醚;卡必醇;乙氧乙氧基乙醇;二甘醇-乙醚;一缩二乙二醇单乙醚;DECS;2-(2-乙氧基乙氧基)乙醇;一缩二乙二醇一乙醚
英文名称
ethoxyethoxyethanol
英文别名
2-(2-ethoxyethoxy)ethanol;diethylene glycol monoethyl ether;transcutol;2-(2-ethoxyethoxy)ethan-1-ol;carbitol
CAS
111-90-0
化学式
C6H14O3
mdl
MFCD00002872
分子量
134.175
InChiKey
XXJWXESWEXIICW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-80 °C
-
沸点:202 °C(lit.)
-
密度:0.999 g/mL at 25 °C(lit.)
-
蒸气密度:4.63 (vs air)
-
闪点:205 °F
-
溶解度:可溶于水
-
LogP:-0.54 at 20℃
-
物理描述:Diethylene glycol monoethyl ether appears as a colorless, slightly viscous liquid with a mild pleasant odor. Flash point near 190°F. Used to make soaps, dyes, and other chemicals.
-
颜色/状态:Colorless liquid
-
气味:Mild, pleasant odor
-
味道:BITTER
-
蒸汽密度:4.62 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.126 mm Hg at 25 °C (est)
-
大气OH速率常数:5.72e-11 cm3/molecule*sec
-
自燃温度:400 °F (204 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
粘度:3.85 mPa.s (=cPs) at 25 °C
-
表面张力:Surface tension: 31.8 mN/m (=dyn/cm) at 25 °C
-
折光率:Index of refraction: 1.4273 at 20 °C/D
-
保留指数:999;982.9;985;985;986;976;979;976
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:9
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:38.7
-
氢给体数:1
-
氢受体数:3
ADMET
代谢
...Carbitol is largely destroyed in body or conjugated with glucuronic acid and excreted as the glucuronate. ...This metabolic peculiarity may explain its lesser toxicity when compared with that of diethyl, methyl and butyl cellosolve.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
干燥的皮肤。
Dry skin.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
Redness.
Redness.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
神经毒素 - 急性溶剂综合征
职业性肝毒素 - 继发性肝毒素:职业环境中潜在毒性效应的判断基于人类摄入或动物实验的中毒案例。
肾毒素 - 该化学品在职业环境中可能对肾脏有毒。
生殖毒素 - 对生殖系统有毒的化学品,包括对后代造成缺陷以及对男性或女性生殖功能的损害。生殖毒性包括发育效应。参见生殖毒性风险评估指南。
Neurotoxin - Acute solvent syndrome
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Nephrotoxin - The chemical is potentially toxic to the kidneys in the occupational setting.
Reproductive Toxin - A chemical that is toxic to the reproductive system, including defects in the progeny and injury to male or female reproductive function. Reproductive toxicity includes developmental effects. See Guidelines for Reproductive Toxicity Risk Assessment.
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
LC50 (大鼠) > 5240 毫克/立方米/4小时
LC50 (rat) > 5240 mg/m3/4H
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
人类暴露研究...当在48小时封闭贴片测试中,将材料以20%的水平测试于石油中时,人类志愿者既没有显示出刺激也没有敏感化的迹象。
/HUMAN EXPOSURE STUDIES/ ... Human volunteers showed neither irritation nor signs of sensitization when the material was tested at a 20 percent level in petroleum for a 48 hour closed patch test.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
为了评估选定的未稀释乙二醇醚与皮肤接触的危害,我们测量了它们在体外通过孤立的人腹部表皮的吸收情况。表皮膜被置于玻璃扩散细胞中,在确定了对三氢化水(tritiated water)的渗透性之后,将过量的未稀释乙二醇醚施加在外表面8小时。通过气相色谱技术定量测定了乙二醇醚在水性受体相中的出现,该受体相沐浴在表皮下方。最后确定了三氢化水的渗透性与初始值进行比较,以确定与乙二醇醚接触引起的表皮屏障功能的任何不可逆改变。2-甲氧基乙醇(EM)最容易被吸收(平均稳态速率2.82 mg/平方厘米/小时),1-甲氧基丙醇-2(PM)的吸收率也相对较高(1.17 mg/平方厘米/小时)。随着分子量的增加或挥发性的降低,单乙二醇醚(EM,2.82 mg/平方厘米/小时;2-乙氧基乙醇,EE,0.796 mg/平方厘米/小时;2-丁氧基乙醇,EB,0.198 mg/平方厘米/小时)的吸收率呈下降趋势,且在二乙二醇系列中也有同样的趋势:2-(2-甲氧基乙氧基)乙醇(DM,0.206 mg/平方厘米/小时);2-(2-乙氧基乙氧基)乙醇(DE,0.125 mg/平方厘米/小时)和2-(2-丁氧基乙氧基)乙醇(DB,0.035 mg/平方厘米/小时)。2-乙氧基乙酯(EEAc)的吸收速率与其母醇EE相似。二乙二醇醚的吸收速率慢于相应的单乙二醇等价物。内在毒性与穿过皮肤的能力相结合,有助于评估与未稀释乙二醇醚接触的危害。
To assist evaluation of the hazards of skin contact with selected undiluted glycol ethers, their absorption across isolated human abdominal epidermis was measured in vitro. Epidermal membranes were set up in glass diffusion cells and, following an initial determination of permeability to tritiated water, excess undiluted glycol ether was applied to the outer surface for 8 hr. The appearance of glycol ether in an aqueous receptor phase bathing the underside of the epidermis was quantified by a gas chromatographic technique. A final determination of tritiated water permeability was compared with initial values to establish any irreversible alterations in epidermal barrier function induced by contact with the glycol ethers. 2-methoxyethanol (EM) was most readily absorbed (mean steady rate 2.82 mg/sq cm/hr), and a relatively high absorption rate (1.17 mg/sq cm/hr) was also apparent for 1-methoxypropan-2-ol (PM). There was a trend of reducing absorption rate with increasing molecular weight or reducing volatility for monoethylene glycol ethers (EM, 2.82 mg/sq cm/hr; 2-ethoxyethanol, EE, 0.796 mg/sq cm/hr; 2-butoxyethanol, EB, 0.198 mg/sq cm/hr) and also within the diethylene glycol series: 2-(2-methoxyethoxy) ethanol (DM, 0.206 mg/sq cm/hr); 2-(2-ethoxyethoxy) ethanol (DE, 0.125 mg/sq cm/hr) and 2-(2-butoxyethoxy) ethanol (DB, 0.035 mg/sq cm/hr). The rate of absorption of 2-ethoxyethyl acetate (EEAc) was similar to that of the parent alcohol, EE. Absorption rates of diethylene glycol ethers were slower than their corresponding monoethylene glycol equivalents. Combination of intrinsic toxicity and ability to pass across skin contribute to assessment of hazards of contact with undiluted glycol ethers.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
When a single /oral/ dose of diethylene glycol monoethyl ether (11.2 mmol) was given to an adult human volunteer (sex and age not specified) about 68% of the dose was excreted in the urine as (2-ethoxyethoxy)acetic acid within 12 hr.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
对家兔进行口服、静脉注射、皮下注射和经皮给药后,研究了其对二乙二醇单乙醚的定量尿排泄。在以5毫升/千克体重的剂量对两只动物进行口服给药后,两只动物在第一天死亡,总排泄量仅为0.8%和0.33%。在以0.5-3.4毫升/千克的剂量水平对15只家兔进行静脉给药后,大部分剂量在第一天排泄(15只中有13只),排泄的百分比随着剂量的增加而增加。在单次给药1.0-3.0毫升/千克后,监测了连续4天的尿排泄。在最初的24小时内排泄量很高,随着剂量的增加,尿液中排泄的剂量百分比也随之增加。在重复每日给药0.16、0.32或0.63毫升/千克体重后,总尿排泄量随着剂量的增加而增加,分别等于4.7%、5.0%和11.6%。
The quantitative urinary excretion of diethylene glycol monoethyl ether was investigated in the rabbit after oral, intravenous, subcutaneous and percutaneous administration. After oral dosing of two animals at a level of 5 mL/kg bw, both animals died during the first day and total excretion was only 0.8% and 0.33%. After intravenous administration to 15 rabbits at dose levels of 0.5-3.4 mL/kg most of the dose was excreted during the first day (for 13/15 rabbits), the percentage excreted tending to increase with dose. After a single parenteral dose of 1.0-3.0 mL/kg, urinary excretion was monitored for up to 4 consecutive days. Excretion was high in the first 24 hr and the total percentage of the dose excreted in urine increased with dose. After repeated daily parenteral doses of 0.16, 0.32 or 0.63 mL/kg bw, total urinary excretion increased with dose and equalled 4.7, 5.0 and 11.6%, respectively.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
The oral administration fo 1503 mg Diethylene glycol monoethyl ether to a normal man resulted in the excretion of 1140 mg of 2-ethoxyethoxyacetic acid, 69 % of the total dose, within 12 hr.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S23,S24/25,S26,S39
-
危险类别码:R36,R20
-
WGK Germany:1
-
海关编码:29094400
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:KK8750000
-
包装等级:Z01
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:1. 储存于阴凉、通风的库房。远离火种和热源,并应与氧化剂、酸类等分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 2. 采用玻璃瓶包装,外用木箱加固,防撞击、防热、防火。按易燃有毒物品规定贮运。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 二乙二醇单乙基醚;二乙二醇乙醚 |
| 化学品英文名称: | Diethylene glycol monoethyl ether;Carbit0l |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 111-90-0 |
| 分子式: | C 6 H 14 O 3 |
| 分子量: | 134.18 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:二乙二醇单乙基醚;二乙二醇乙醚 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 |
| 健康危害: | 动物实验引起麻醉作用及肾脏损害。对眼刺激不明显。对皮肤几无刺激性,未见职业性危害。 |
| 环境危害: | |
| 燃爆危险: | 本品可燃。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用流动清水冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水或生理盐水冲洗,就医。 |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。就医。 |
| 食入: | 给饮足量温水,催吐,就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或与氧化剂接触,有引起燃烧的危险。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 94 |
| 自燃温度(℃): | 引燃温度(℃):无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿一般作业工作服。尽可能切断泄漏源。防止流入下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,全面通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴过滤式防毒面具(半面罩),戴防化学品手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、酸类接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、酸类等分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC;未制订标准前苏联MAC:未制订标准美国TLV—TWA:未制订标准美国 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 高浓度接触时,应该佩戴防毒面具。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 必要时戴防化学品手套。 |
| 其他防护: | 工作现场严禁吸烟。避免长期反复接触。定期体检。 |
| 第九部分:理化特性 |
| 外观与性状: | 具有微弱芳香气味和苦味的无色液体。 |
| pH: | |
| 熔点(℃): | -76 |
| 沸点(℃): | 201.9 |
| 相对密度(水=1): | 0.99(20℃) |
| 相对蒸气密度(空气=1): | 4.62 |
| 饱和蒸气压(kPa): | 0.017(25℃) |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 94 |
| 引燃温度(℃): | 引燃温度(℃):无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 6 H 14 O 3 |
| 分子量: | 134.18 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 可混溶于丙酮、苯、氯仿、乙醇、乙醚。 |
| 主要用途: | 用作树胶、喷漆、染料等的溶剂,也作为稀释剂和某些化学中间产物。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强酸、酰基氯、酸酐。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:5540mg/kg(大鼠经口);6580mg/kg(小鼠经口) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂、酸类等混装混运。船运时,应与机舱、电源、火源等部位隔离。公路运输时要按规定路线行驶。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 3 |
| MSDS修改日期: | 年月日 |
制备方法与用途
二乙二醇乙醚在常温常压下为无色透明液体,具有中等程度的令人愉快气味。该物质广泛用作溶剂,适用于硝酸纤维素、树脂、喷漆和染料等领域,并作为高沸点溶剂及稀释剂。在精细化工领域,它可用于汽车引擎清洗剂配方;在有机合成中,则可通过羟基的酯化反应或亲核取代反应引入到功能材料分子结构中。
毒性 根据FAO/WHO(2001年),二乙二醇乙醚未规定ADI值。
化学性质 无色、吸水性稳定的液体,有中等程度令人愉快的气味。它能与水、丙酮、苯、氯仿、乙醇、乙醚和吡啶等物质混溶。
用途 主要用于油漆、油墨中的互溶剂以及载体溶剂(特别是在伊斯兰国家作为柑橘香精的乙醇代用品)。此外,该品还可作为高沸点溶剂,用于纤维素、树脂、树胶、涂料、印刷用油墨、染料的溶剂;矿物油-皂和矿物油-硫化油混合物的互溶剂;非油漆着色剂;纤维印染剂;清漆和油漆的稀释剂;以及溶剂,并用于清漆和塑料制备。
生产方法
- 环氧乙烷法:先往反应器中加入无水乙醇、三氟化硼-乙醚溶液,再滴加环氧乙烷。当反应温度升至45℃时,冷却并控制反应温度在70℃以下。反应完成后,经中和和减压蒸馏,收集90℃(0.1MPa)以下的馏分,即得成品。
- 二甘醇法:由二甘醇与硫酸二乙酯反应制得。
类别 可燃液体
毒性分级 低毒
急性毒性 口服- 大鼠 LD50: 5500 毫克/ 公斤;小鼠 LD50: 6600 毫克/ 公斤
刺激数据 皮肤- 兔 500 毫克/24小时 轻度;眼睛- 兔 125 毫克 轻度
可燃性危险特性 遇明火、高温或强氧化剂可燃,燃烧时排放刺激烟雾
储运特性 包装完整,轻装轻卸。库房需保持通风,并远离明火和高温,与氧化剂分开存放。
灭火剂 泡沫、干粉、二氧化碳
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二乙二醇甲乙醚 diethylene glycol methyl ethyl ether 1002-67-1 C7H16O3 148.202 四乙二醇二甲醚 Tetraethylene glycol dimethyl ether 143-24-8 C10H22O5 222.282 三甘醇二甲醚 Triethylene glycol dimethyl ether 112-49-2 C8H18O4 178.229 二乙二醇 diethylene glycol 111-46-6 C4H10O3 106.122 乙二醇乙醚 2-ethoxy-ethanol 110-80-5 C4H10O2 90.1222 三乙二醇单丁醚 butyltriethyleneglycol 143-22-6 C10H22O4 206.282 1-[2-(2-甲氧基乙氧基)乙氧基]丁烷 1-<2-(2-Methoxyethoxy)ethoxy>butan 7382-32-3 C9H20O3 176.256 3,6,9,12-四氧杂十六烷-1-醇 Tetraaethylenglykol-mono-butylaether 1559-34-8 C12H26O5 250.335 二乙二醇丁醚 Diethylene glycol monobutyl ether 112-34-5 C8H18O3 162.229 二乙二醇单乙基醚醋酸酯 diethylene glycol monoethyl ether acetate 112-15-2 C8H16O4 176.213 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二乙二醇二乙醚 3,6,9-trioxaundecane 112-36-7 C8H18O3 162.229 二乙二醇甲乙醚 diethylene glycol methyl ethyl ether 1002-67-1 C7H16O3 148.202 三缩四乙二醇 Tetraethylene glycol 112-60-7 C8H18O5 194.228 3,6,9,12-四氧十四烷-1-醇 3,6,9,12-tetraoxatetradecan-1-ol 5650-20-4 C10H22O5 222.282 三乙二醇乙醚 monoethyl ether of triethylene glycol 112-50-5 C8H18O4 178.229 3,6,9,12,15-五氧杂十七烷 tetraethylene glycol diethyl ether 4353-28-0 C12H26O5 250.335 —— 3,6,9,12-tetraoxatetradecane 4499-99-4 C10H22O4 206.282 3,6,9,12,15,18-六氧杂二十烷 1,14-diethoxy-3,6,9,12-tetraoxa-tetradecane 23601-39-0 C14H30O6 294.389 —— <2-Ethoxy-ethyl>-<2-hydroxy-methoxy-ethyl>-ether —— C7H16O4 164.202 [2-(2-乙氧基乙氧基)乙氧基]-乙烯 2-[2-(ethoxy)ethoxy]ethyl vinyl ether 10143-53-0 C8H16O3 160.213 —— triethylene glycol ethyl vinyl ether 87647-92-5 C10H20O4 204.266 3-[2-(2-乙氧基乙氧基)乙氧基]丙-1-炔 (3-(2-(2-ethoxy)ethoxy)ethoxy)-1-propyne 89635-80-3 C9H16O3 172.224 —— 1-chloro-2,5,8-trioxadecane 85232-17-3 C7H15ClO3 182.647 —— O-ethoxyethoxyethylhydroxylamine 54149-51-8 C6H15NO3 149.19 2-(2-(2-(2-乙氧基乙氧基)乙氧基)乙氧基)乙基溴 2-(2-(2-(2-ethoxyethoxy)ethoxy)ethoxy)ethyl bromide 850143-14-5 C10H21BrO4 285.178 1,4-二氧六环 1,4-dioxane 123-91-1 C4H8O2 88.1063 —— 1,13-diethoxy-3,6,8,11-tetraoxa-tridecane 42598-91-4 C13H28O6 280.362 2-(2-乙氧基乙氧基)乙基溴 1-bromo-2-(2-ethoxyethoxy)ethane 54550-36-6 C6H13BrO2 197.072 2-(2-乙氧基乙氧基)-乙胺 2-(2-ethoxyethoxy)ethanamine 23702-88-7 C6H15NO2 133.191 1-(2-氯乙氧基)-2-乙氧基乙烷 1-[2-(ethoxy)ethoxy]-2-chloroethane 41771-35-1 C6H13ClO2 152.621 —— 2-(2-ethoxyethoxy)acetaldehyde 125117-60-4 C6H12O3 132.159 —— 2-butoxyethyl 2-ethoxyethyl ether 3895-17-8 C10H22O3 190.283 3-(2-甲氧基乙氧基)-1-丙烯 3-(2-methoxyethoxy)prop-1-ene 18854-48-3 C6H12O2 116.16 1-(2-碘乙氧基)-2-甲氧基乙烷 2-(2-methoxyethoxy)ethyl iodide 104539-21-1 C5H11IO2 230.046 —— 3,6,9-trioxaundecanoic acid 7743-98-8 C8H16O5 192.212 二乙二醇单乙基醚醋酸酯 diethylene glycol monoethyl ether acetate 112-15-2 C8H16O4 176.213 —— 3-(2-(2-Ethoxyethoxy)ethoxy)propan-1-amine 26383-45-9 C9H21NO3 191.271 —— β-ethoxyethoxyacetic acid 7743-94-4 C6H12O4 148.159 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:用醚基官能化的1,3-二取代脲是有效的可溶性环氧化物水解酶抑制剂,具有改善的药代动力学特性。摘要:可溶性环氧水解酶(sEH)是用于治疗高血压和炎症的治疗靶标。被醚基官能化的1,3-二取代脲是有效的sEH抑制剂。然而,它们相对较低的代谢稳定性导致不良的药代动力学性质。为了提高其生物利用度,我们研究了在醚功能上掺入各种极性基团对抑制能力,物理性质,体外代谢稳定性和药代动力学性质的影响。结构-活性关系研究表明,脲基团和醚官能团之间的疏水性连接基对于保持其效能是必要的。此外,具有极性基团的脲-醚抑制剂,例如二甘醇或吗啉,可显着改善其物理性质和代谢稳定性,而不会丧失任何抑制效力。此外,使用所得抑制剂在鼠和犬模型中获得了改善的药代动力学性质。这些发现将有助于在高血压和发炎的动物模型中使用sEH抑制剂。DOI:10.1021/jm070705c
-
作为产物:参考文献:名称:LOTXOV, V. A.;LANDA, I. V.;MALYUSOV, V. A.;KULOV, N. N., 14 MENDELEEV. SEZD PO OBSHCH. I PRIKL. XIMII, M.,(1989) S. 284摘要:DOI:
-
作为试剂:描述:参考文献:名称:The ortho-Claisen Rearrangement. IV. The Rearrangement of X-Cinnamyl p-Tolyl Ethers1摘要:DOI:10.1021/ja01479a026
文献信息
-
Novel processes for the preparation of adenosine compounds and intermediates thereto申请人:——公开号:US20030069423A1公开(公告)日:2003-04-10Novel processes for the preparation of adenosine compounds and intermediates thereto. The adenosine compounds prepared by the present processes may be useful as cardiovascular agents, more particularly as antihypertensive and anti-ischemic agents, as cardioprotective agents which ameliorate ischemic injury or myocardial infarct size consequent to myocardial ischemia, and as an antilipolytic agents which reduce plasma lipid levels, serum triglyceride levels, and plasma cholesterol levels. The present processes may offer improved yields, purity, ease of preparation and/or isolation of intermediates and final product, and more industrially useful reaction conditions and workability.
-
[EN] S-NITROSOMERCAPTO COMPOUNDS AND RELATED DERIVATIVES<br/>[FR] COMPOSÉS DE S-NITROSOMERCAPTO ET DÉRIVÉS APPARENTÉS申请人:GALLEON PHARMACEUTICALS INC公开号:WO2009151744A1公开(公告)日:2009-12-17The present invention is directed to mercapto-based and S- nitrosomercapto-based SNO compounds and their derivatives, and their use in treating a lack of normal breathing control, including the treatment of apnea and hypoventilation associated with sleep, obesity, certain medicines and other medical conditions.本发明涉及基于巯基和S-亚硝基巯基的SNO化合物及其衍生物,以及它们在治疗正常呼吸控制缺失方面的用途,包括治疗与睡眠、肥胖、某些药物和其他医疗状况相关的呼吸暂停和低通气。
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
[EN] HORMONE RECEPTOR MODULATORS FOR TREATING METABOLIC MUTAGENIC AND FIBROTIC CONDITIONS AND DISORDERS<br/>[FR] MODULATEURS DU RÉCEPTEUR HORMONAL POUR LE TRAITEMENT D'ÉTATS ET DE TROUBLES MÉTABOLIQUES MUTAGÈNES ET FIBROTIQUES申请人:ARDELYX INC公开号:WO2019055808A1公开(公告)日:2019-03-21The invention relates to activators of FXR useful in the treatment of autoimmune disorders, liver disease, intestinal disease, kidney disease, cancer, and other diseases in which FXR plays a role, having the Formula (I): wherein L1, A, X1, X2, Y1, Y2, Y3, Y4, R1, R2, and R3 are described herein.这项发明涉及FXR的激活剂,可用于治疗自身免疫性疾病、肝病、肠道疾病、肾脏疾病、癌症以及FXR发挥作用的其他疾病,其化学式为(I):其中L1、A、X1、X2、Y1、Y2、Y3、Y4、R1、R2和R3如本文所述。
表征谱图
-
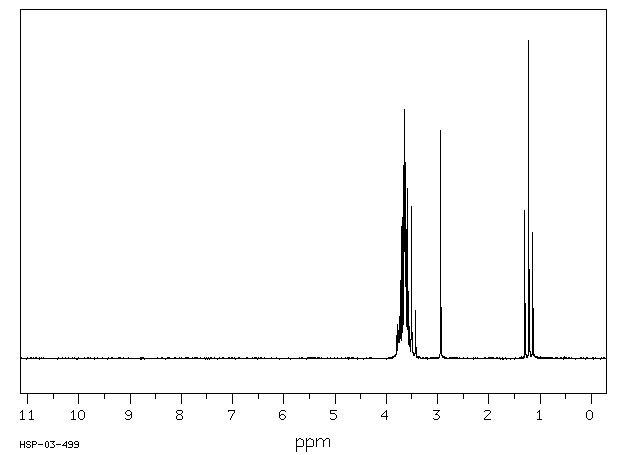
氢谱1HNMR
-
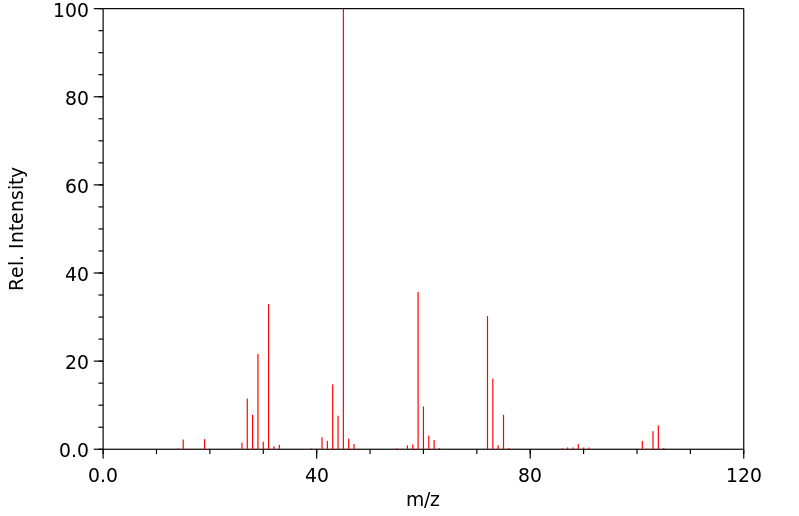
质谱MS
-
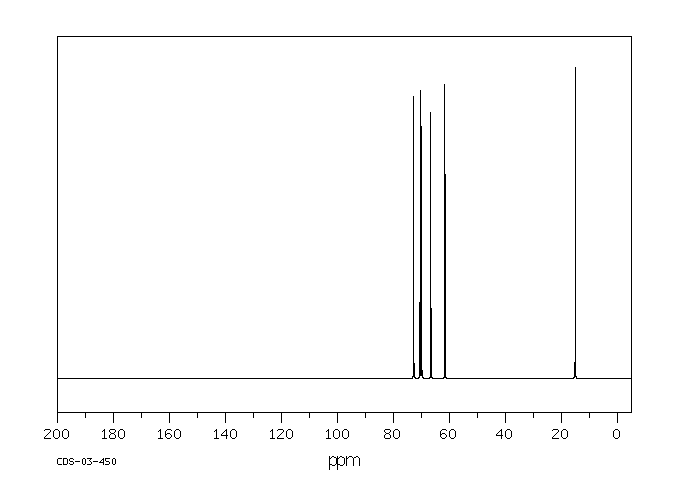
碳谱13CNMR
-
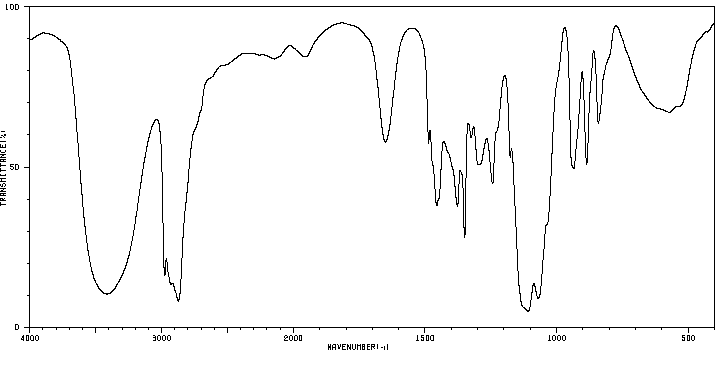
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷