尼泊金异丁酯 | 4247-02-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:76°C
-
沸点:302.3±15.0 °C(Predicted)
-
密度:1.105±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:3.43 at 23℃
-
保留指数:1699
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S22,S24/25
-
危险类别码:R51/53
-
WGK Germany:2
-
海关编码:2918290000
-
危险品运输编号:UN 3077 9/PG 3
-
RTECS号:DH2247000
-
储存条件:避光、存于通风干燥处,并密封保存。
SDS
Section I.Chemical Product and Company Identification
Chemical Name Isobutyl 4-Hydroxybenzoate
Portland OR
Synonym 4-Hydroxybenzoic Acid Isobutyl Ester;
Isobutylparaben
Chemical Formula C11H14O3
CAS Number 4247-02-3
Section II. Composition and Information on Ingredients
Toxicology Data
Chemical Name CAS Number Percent (%) TLV/PEL
Min. 99.0 (T) Not available. Not available.
Isobutyl 4-Hydroxybenzoate 4247-02-3
Section III. Hazards Identification
Acute Health Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Skin contact may result in sensitization. Always cover all exposed skin with an impermeable layer and use proper eye
protection. A OSHA/MSHA approved dust and vapor respirator is required when working with this material.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
Eye Contact
minutes. Get medical attention.
In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
Skin Contact
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
May be combustible at high temperature. Auto-Ignition
Flammability
Flammable Limits Not available.
Flash Points Not available.
Combustion Products These products are toxic carbon oxides (CO, CO2).
Fire Hazards
Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
Isobutyl 4-Hydroxybenzoate
Section VI. Accidental Release Measures
Spill Cleanup Irritating material. Skin sensitizing material.
Use a shovel to put the material into a convenient waste disposal container. Consult federal, state, and/or local authorities for
Instructions
assistance on disposal.
Section VII. Handling and Storage
Handling and Storage IRRITANT. SKIN SENSITIZER. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the
container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe dust.
Information
Section VIII. Exposure Controls/Personal Protection
Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended
Engineering Controls
exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants
below the exposure limit.
Splash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
Personal Protection
specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Physical state @ 20°C Solid. (Crystal, powder. Whtie - almost Solubility
Soluble in methanol;
white.)
Very soluble in ethanol, ether, acetone;
Very slightly soluble in water.
Not available.
Specific Gravity
Molecular Weight 194.23 Partition Coefficient LOG Pow: 3.11
Boiling Point Not available. Vapor Pressure Not applicable.
Melting Point 76°C (168.8°F) Vapor Density Not available.
Not available. Not available.
Refractive Index Volatility
Critical Temperature Not available. Odor Not available.
Not available. Not available.
Viscosity Taste
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities Reactive with strong oxidizing agents.
Section XI. Toxicological Information
RTECS Number DH2247000
Eye Contact. Ingestion. Inhalation.
Routes of Exposure
Not available.
Toxicity Data
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye
Acute Toxic Effects
is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Skin contact may result in sensitization. Always cover all exposed skin with an impermeable layer and use proper eye
protection. A OSHA/MSHA approved dust and vapor respirator is required when working with this material.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Section XII. Ecological Information
Not available.
Ecotoxicity
Environmental Fate Not available.
Continued on Next Page
Isobutyl 4-Hydroxybenzoate
Section XIII. Disposal Considerations
Waste Disposal Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
Not a DOT controlled material (United States).
DOT Classification
PIN Number Not applicable.
Proper Shipping Name Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification On DSL.
(Canada)
EINECS Number (EEC) 224-208-8
EEC Risk Statements R36/37/38- Irritating to eyes, respiratory system and skin.
R43- May cause sensitization by skin contact.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
防腐剂尼泊金异丁酯又称对羟基苯甲酸酯。由于其高效、低毒、广谱和易配伍的优点,除了作为许多发达国家的主流食品防腐剂外,还广泛应用于日化、医药及饲料行业中的防腐。
应用尼泊金异丁酯(对羟基苯甲酸酯)能有效抑制粮食霉菌、酵母和细菌的生长。它通过破坏微生物细胞膜使蛋白质变性,并抑制其呼吸酶系和电子传递酶系的活性。这种抑菌作用在较宽的pH值范围内都有良好的效果,因此被广泛应用于食品、饲料、化妆品、日用化工品及医药等行业,是国际上采用的安全有效的防腐剂之一,也是我国重点发展的食品防腐剂。
鉴别试验尼泊金异丁酯与“对羟基苯甲酸乙酯(07009)”的鉴别试验中1、2条相同。
含量分析含量分析方法与“对羟基苯甲酸乙酯(07009)”的方法相同,只是试样无需预经干燥。每mL lmol/L氢氧化钠液相当于对羟基苯甲酸异丁酯(C11H14O3) 194.2mg。
毒性尼泊金异丁酯的LD50为8.39g/kg(大鼠,经口)。
使用限量日本规定的最大使用量(以对羟基苯甲酸计,1g相当于0.711g对羟基苯甲酸,g/kg)如下:
- 酱油:0.25g/L(本品0.35)
- 食用醋:0.1g/L(本品0.14)
- 清凉饮料及糖浆:0.1(本品0.14)
- 水果调味酱:0.2(本品0.28)
- 果蔬:0.012(本品0.016)
尼泊金异丁酯为无色细小晶体或白色结晶性粉末,无臭。难溶于水(0.035g/100ml,25℃),易溶于乙醇、冰醋酸、丙二醇和丙酮。
用途尼泊金异丁酯作为防腐剂和防霉剂被广泛应用。虽然它很少单独使用,但常与其他类型的对羟基苯甲酸酯(如异丙酯、丁酯等)混合,用作水包油型乳化剂。与单纯丁酯相比,其溶解度增加2~3倍,并易于应用于酱油中。
用途尼泊金异丁酯也作为食品保存剂和防霉剂使用。目前日本允许使用的对羟基苯甲酸酯类包括乙酯、丙酯、异丙酯、丁酯、异丁酯及另丁酯等六种,其中以丁酯的抗菌作用最强。通常情况下,构成酯的醇碳原子数越大,其抗菌作用越强,而毒性则相反,碳原子数越小,毒性越大。尼泊金异丁酯对各种霉菌和酵母具有抑制或杀灭作用,并且不太受pH值的影响。其在酱油、醋、清凉饮料、果子露、水果沙司及果蔬(限表皮部分)中的使用量为对羟基苯甲酸的1.4倍。
生产方法尼泊金异丁酯由对羟基苯甲酸与异丁醇酯化而成。在硫酸存在下进行酯化反应,以苯或甲苯作为溶剂,在加热回流条件下生成对羟基苯甲酸异丁酯。按《日本食品添加物公定书》规定,产品纯度应在99%以上。尼泊金异丁酯的毒性较小,鼠口服LD50为8.38g/kg。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对羟基苯甲酸 4-hydroxy-benzoic acid 99-96-7 C7H6O3 138.123 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— isobutyl p-(3-bromo)-propyloxy benzoate 160834-75-3 C14H19BrO3 315.207 —— 2-Methylpropyl 4-acetyloxybenzoate 27801-52-1 C13H16O4 236.268 —— 4-(5-bromopentoxy)isobutylbenzoate 147539-06-8 C16H23BrO3 343.261 4-乙酰氧基苯甲酸丙酯 n-propyl 4-acetoxybenzoate 27739-13-5 C12H14O4 222.241 —— 3-(4-isobutoxycarbonyl)phenoxy-1-bromoacetone 1025828-97-0 C14H19BrO4 331.206 —— isobutyl-p-(3-bromo-2-oxo)propoxy benzoate 147539-04-6 C14H17BrO4 329.191 —— 4-isobutoxycarbonylphenoxy-2,3-epoxypropane 147539-05-7 C14H18O4 250.295 —— 4-Acetoxybenzoic acid butyl ester 27739-14-6 C13H16O4 236.268 乙基4-乙酰氧基苯甲酸酯 ethyl 4-acetoxybenzoate 13031-45-3 C11H12O4 208.214 —— Isopropyl 4-acetoxybenzoate 27739-15-7 C12H14O4 222.241 4-乙酰氧基苯甲酸甲酯 methyl 4-acetoxybenzoate 24262-66-6 C10H10O4 194.187 —— Benzyl-p-acetoxy-benzoat 54835-09-5 C16H14O4 270.285 - 1
- 2
反应信息
-
作为反应物:描述:尼泊金异丁酯 在 2,6-二甲基吡啶 、 sodium hydride 作用下, 以 乙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 2.0h, 生成 (R)-2-<3-<(4-isobutoxycarbonyl)phenoxy>-2-hydroxypropylthio>-4,5-diphenylimidazole参考文献:名称:Novel aryloxyalkylthioimidazoles as inhibitors of acyl-CoA: cholesterol-O-acyltransferase摘要:A series of aryloxyalkylthioimidazoles have been synthesized and evaluated for their ability,to interfere with the enzyme acyl-CoA (cholesterol-O-acyltransferase) (ACAT, EC 2.3.1.26). Most of the molecules possessed a good in vitro ACAT inhibitory activity with IC50 values ranging between 0.1 and 2.0 mu M Some of them, eg, 2-{5-[(4-isobutoxycarbonyl)phenoxy]-pentylthio} -4,5-diphenylimidazole 13, 2-{3-[(4-isobutoxycarbonyl)phenoxy]-2-oximinopropylthio}-4,5-diphenylimidazole 21, 2-{3-[(4-isobutoxycarbonyl)phenoxy]-2-hydrazonecarboxamidepropylthio) -4,5-diphenylimidazole 26, 2-[5-(2-pyridoxy)-pentylthio]-4,5-diphenylimidazole 40 and 2-{5-[(3,5-diterbutyl-4-hydroxy)phenylthio]pentylthio}-4,5-diphenylimidazole 42, were more potent (range of activity 10-90 nM). They were also more potent with respect to the reference CI-976. When administered orally in hyperlipemic rats, at 10 and 50 mg/kg doses, some representative compounds, like 2-{3-[(4-isobutoxycarbonyl)phenoxy]-2-hydroxypropylthio}-4,5-diphenylimidazole 1, 13 and 26, reduced VLDL/LDL-associated cholesterol levels by 30-50% and increased HDL cholesterol levels by 15-50%. In addition, liver accumulation of esterified cholesterol was counteracted (50-80% reduction) and liver ACAT ex vivo activity was decreased by 70-85%. Finally, the good efficacy displayed in an endogenous model of hypertriglyceridemia strongly supports the hypothesis of a good systemic availability, which constitutes one of the principal properties of a valuable ACAT inhibitor.DOI:10.1016/0223-5234(96)88207-9
-
作为产物:参考文献:名称:使用 Houben-Hoesch 反应从富含同位素的酚中合成 13C 标记的对羟基苯甲酸酯摘要:对羟基苯甲酸酯是多种消费品中的抗菌添加剂。然而,在废水消毒过程中由对羟基苯甲酸酯形成的卤代化合物是一个潜在的环境问题。为了识别这些转化产物并研究它们的形成机制,开发了一种在苯环内特定位置用稳定同位素碳 13 标记对羟基苯甲酸乙酯的合成路线。这种有效的两步法从市售的13 C 标记的苯酚开始,包括 (1) 通过与三氯乙腈的 Houben-Hoesch 反应对苯酚进行初始酰化,然后 (2) 对所得三氯甲基酮进行改进的卤仿反应,得到对应13C 标记的对羟基苯甲酸乙酯的总产率为 65%–80%。还研究了改进的卤仿反应的范围,允许合成衍生自伯醇或仲醇的其他对羟基苯甲酸酯,包括13 C-和氘标记的酯。此外,4-羟基苯甲酸可以直接由常见的三氯甲基酮中间体在用氢氧化锂处理后形成。该方案补充了现有的制备13 C 标记的对羟基苯甲酸酯衍生物的方法,并提供了在 Houben-Hoesch 反应(形成对二取代产物)DOI:10.1002/jlcr.3992
文献信息
-
[EN] ANTIBACTERIAL COMPOUNDS<br/>[FR] COMPOSÉS ANTIBACTÉRIENS申请人:MASSACHUSETTS GEN HOSPITAL公开号:WO2019199979A1公开(公告)日:2019-10-17The present application provides compounds of formula: Methods of using these compounds for killing bacterial growth and treating bacterial infections are also provided.本申请提供了以下化合物的公式:还提供了使用这些化合物杀灭细菌生长和治疗细菌感染的方法。
-
Vitamin D derivative having substituent at 2beta-position申请人:CHUGAI SEIYAKU KABUSHIKI KAISHA公开号:US20020045772A1公开(公告)日:2002-04-18A steroid intermediate has the formula (II): 1 wherein A denotes alkylene of 2 to 10 carbons; X denotes halogen; R a , R b and R c denote, independently hydrogen or hydroxyl protecting group; and R p , R q , R y and R z are such that R p and R q together form a double bond between the 5-position and the 6-position and R y and R z together form a double bond between the 7-position and the 8-position, or R q and R y together form a double bond between the 6-position and the 7-position and R p and R z are bound to a dienophile capable of protecting conjugated double bonds.一种甾体中间体具有公式(II):1,其中A代表2至10个碳原子的烷基;X代表卤素;Ra、Rb和Rc分别独立表示氢或羟基保护基团;而Rp、Rq、Ry和Rz是这样的,即Rp和Rq共同在5位和6位之间形成一个双键,Ry和Rz共同在7位和8位之间形成一个双键,或者Rq和Ry共同在6位和7位之间形成一个双键,而Rp和Rz则与能保护共轭双键的二烯烃基团结合。
-
Extracts of Isochrysis sp.申请人:Herrmann Martina公开号:US20100080761A1公开(公告)日:2010-04-01The present invention relates to extracts of Isochrysis sp., preferably Tahitian Isochrysis, its cosmetic, dermatological and/or therapeutic uses and compositions and cosmetic, dermatological or therapeutic products comprising such an extract of Isochrysis sp., preferably Tahitian Isochrysis.
-
[EN] TRIAZINE DERIVATIVES AS UV ABSORBERS<br/>[FR] DERIVES DE LA TRIAZINE UTILISES COMME ABSORBANTS U.V.申请人:CIBA SC HOLDING AG公开号:WO2004064797A1公开(公告)日:2004-08-05The present invention relates to new compounds of formula (I), wherein R1, R2, R3, R4, R5, R6, R7, and R8 independently from each other are hydrogen; C1-C18alkyl; C2-C18alkenyl; C5-C7,cycloalkyl; C1-C6alkylene-C5- C7,cycloalkyl; R9 is hydrogen; C1-C18alkyl; C2-C18alkenyl; C5-C7cycloalkyl; C1-C6alkylene-C5-C7cycloalkyl; C6-C10aryl; A is-S-; -O- or -NR10-, wherein R10 has the same meanings as R9; X is COOR11; CONR12R13; SO3,R14; or SO2NR15R16, wherein R11, R12,R13, R14,R15, and R16, have independently from each other the same meanings as R9; to their preparation and to their use as UV absorbers in cosmetic and pharmaceutical formulations.本发明涉及公式(I)的新化合物,其中R1、R2、R3、R4、R5、R6、R7和R8彼此独立地是氢;C1-C18烷基;C2-C18烯基;C5-C7环烷基;C1-C6烷基-C5-C7环烷基;R9是氢;C1-C18烷基;C2-C18烯基;C5-C7环烷基;C1-C6烷基-C5-C7环烷基;C6-C10芳基;A是-S-;-O-或-NR10-,其中R10具有与R9相同的含义;X是COOR11;CONR12R13;SO3R14;或SO2NR15R16,其中R11、R12、R13、R14、R15和R16彼此独立地具有与R9相同的含义;以及它们的制备和它们作为化妆品和药用配方中的紫外线吸收剂的用途。
-
AhR mediators申请人:Krutmann Jean公开号:US20090028804A1公开(公告)日:2009-01-29The invention relates to a method for finding and assessing agonists [and] antagonists of the aryl hydrocarbon receptor (Ah receptor; AhR), to the agonists and antagonists themselves and to uses thereof.这项发明涉及一种用于寻找和评估芳香族羟基化合物受体(Ah受体;AhR)的激动剂和拮抗剂的方法,以及这些激动剂和拮抗剂本身及其用途。
表征谱图
-
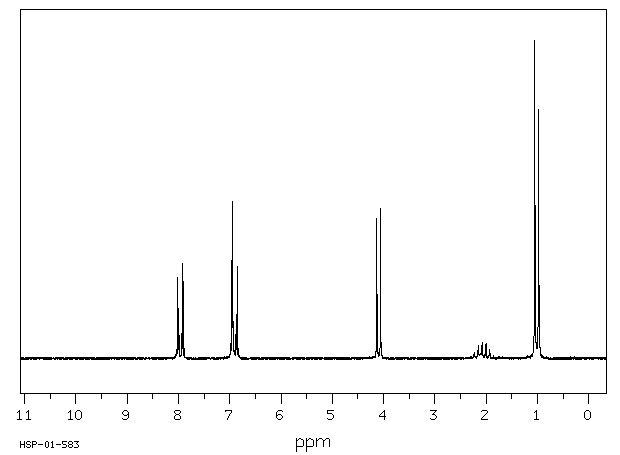
氢谱1HNMR
-
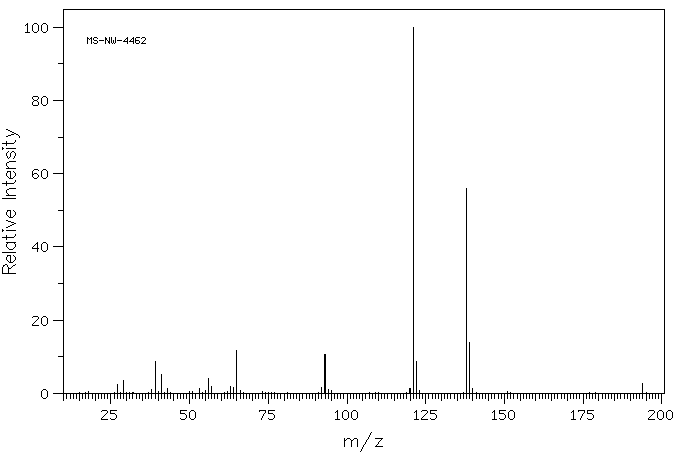
质谱MS
-
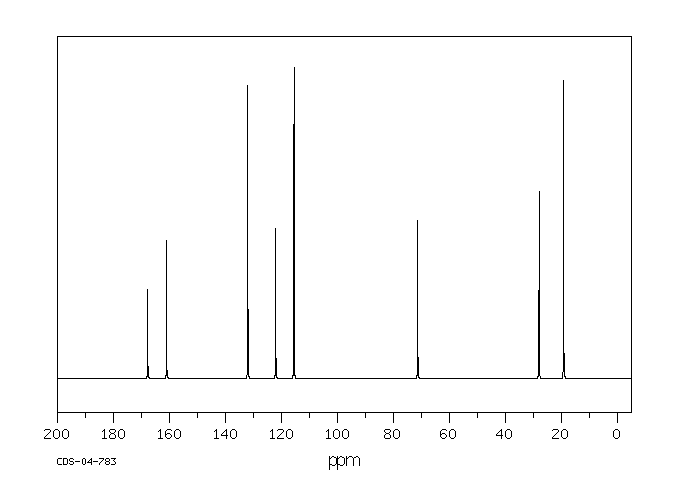
碳谱13CNMR
-
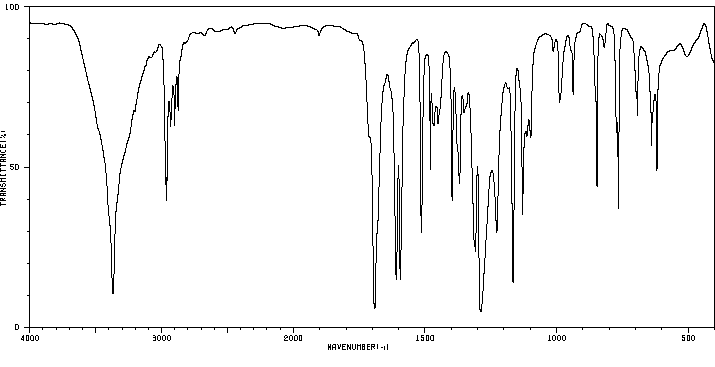
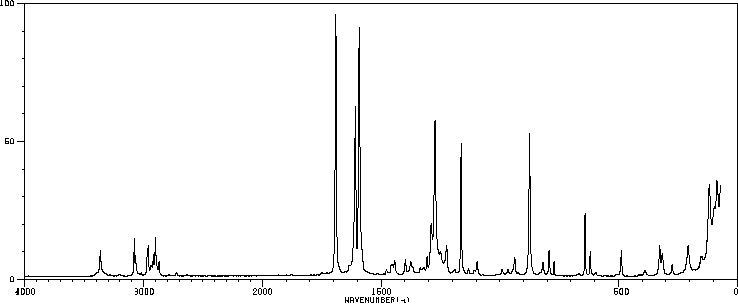
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息