尼泊金异丙酯 | 4191-73-5
中文名称
尼泊金异丙酯
中文别名
4-羟基苯甲酸异丙酯;羟苯异丙酯;对羟基苯甲酸异丙酯
英文名称
isopropyl 4-hydroxybenzoate
英文别名
isopropylparaben;propan-2-yl 4-hydroxybenzoate
CAS
4191-73-5
化学式
C10H12O3
mdl
MFCD00016468
分子量
180.203
InChiKey
CMHMMKSPYOOVGI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:84-86°C
-
沸点:160 °C / 5mmHg
-
密度:1.132±0.06 g/cm3(Predicted)
-
溶解度:易溶于氯仿、乙酸乙酯。
-
LogP:2.316 at 25℃
-
稳定性/保质期:
通常对水没有危害,若无政府许可,请勿将材料排入周围环境。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
RTECS号:DH2250000
-
WGK Germany:2
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
Section I.Chemical Product and Company Identification
Chemical Name Isopropyl 4-Hydroxybenzoate
[for Biochemical Research]
Portland OR
Benzoic acid, 4-hydroxy-, 1-methylethyl ester (CA
Synonym
INDEX NAME);
4-Hydroxybenzoic Acid Isopropyl Ester;
Isopropylparaben
Chemical Formula C10H12O3
4191-73-5
CAS Number
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
Isopropyl 4-Hydroxybenzoate 4191-73-5 Min. 99.0 (T) Not available. Mouse LD50 (subcutaneous) 1900
[for Biochemical Research]
mg/kg
Section III. Hazards Identification
Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Acute Health Effects
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Skin contact may result in sensitization. Always cover all exposed skin with an impermeable layer and use proper eye
protection. A OSHA/MSHA approved dust and vapor respirator is required when working with this material.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
Inhalation
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Ingestion
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
May be combustible at high temperature. Auto-Ignition
Flammability
Flash Points Flammable Limits Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO2).
Fire Hazards Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
Isopropyl 4-Hydroxybenzoate
[for Biochemical Research]
Section VI. Accidental Release Measures
Spill Cleanup Harmful material. Irritating material. Skin sensitizing material.
Use a shovel to put the material into a convenient waste disposal container. Consult federal, state, and/or local authorities for
Instructions
assistance on disposal.
Section VII. Handling and Storage
HARMFUL. IRRITANT. SKIN SENSITIZER. Keep away from heat. Mechanical exhaust required. When not in use, tightly
Handling and Storage
seal the container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe dust.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended
Engineering Controls
exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants
below the exposure limit.
Splash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
Personal Protection
specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solid. (Powder, flake. White.) Solubility
Physical state @ 20°C Soluble in methanol.
Not available.
Specific Gravity
180.20
Molecular Weight Partition Coefficient LOG Pow: 2.39
Boiling Point 160°C (320°F) @ 5 mmHg Vapor Pressure Not applicable.
86°C (186.8°F) Not available.
Melting Point Vapor Density
Refractive Index Not available. Volatility Not available.
Not available. Not available.
Critical Temperature Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
This material is stable if stored under proper conditions. (See Section VII for instructions)
Stability
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
DH2250000
RTECS Number
Routes of Exposure Eye Contact. Ingestion. Inhalation.
Mouse LD50 (subcutaneous) 1900 mg/kg
Toxicity Data
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Acute Toxic Effects
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye
is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Skin contact may result in sensitization. Always cover all exposed skin with an impermeable layer and use proper eye
protection. A OSHA/MSHA approved dust and vapor respirator is required when working with this material.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Continued on Next Page
Isopropyl 4-Hydroxybenzoate
[for Biochemical Research]
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
Not a DOT controlled material (United States).
DOT Classification
Not applicable.
PIN Number
Proper Shipping Name Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
On DSL.
(Canada)
EINECS Number (EEC) 224-069-3
EEC Risk Statements R20/21/22- Harmful by inhalation, in contact with skin and if swallowed.
R36/37/38- Irritating to eyes, respiratory system and skin.
R43- May cause sensitization by skin contact.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
简介
4-羟基苯甲酸异丙酯是一种羧酸酯类衍生物,广泛应用于酱油、醋、清凉饮料(不含汽水)、果品调味剂、水果及蔬菜、腌制品等领域。此外,它还被用于食品、化妆品和医药的防腐、防霉和杀菌。
应用4-羟基苯甲酸异丙酯(尼泊金异丙酯)通常与其他酯类如异丙酯、丁酯等混合使用,作为水包油型乳化剂。与单纯丁酯相比,它的溶解度增加2~3倍,并且更容易应用于酱油中。
尼泊金异丙酯与对羟基苯甲酸丁酯一样,可用作食品的保存剂和防霉剂。日本允许使用的对羟基苯甲酸酯类包括乙酯、丙酯、异丙酯、丁酯、异丁酯和邻丁酯等6种,其中丁酯的抗菌作用最强。一般来说,构成酯的醇分子中的碳原子数越大,其抗菌能力越强,而毒性则相反;碳原子数较小的化合物毒性较大。该物质对各种霉菌和酵母有抑制或杀灭作用,并且不太受pH值的影响。它限用于酱油、醋、清凉饮料、果子露、水果沙司以及某些水果和蔬菜(仅限于表皮部分),其用量相当于对羟基苯甲酸的1.4倍。
制备由对羟基苯甲酸与异丁醇酯化而得。该反应在硫酸存在下进行,以苯或甲苯为溶剂,在加热回流条件下完成。根据《日本食品添加物公定书》的规定,产品的纯度应超过99%。尼泊金异丙酯的毒性较低,小鼠口服LD50值为8.38g/kg。
鉴别试验与“对羟基苯甲酸乙酯(07009)”进行鉴别试验中的1、2条相同。
含量分析与“07009”中所述方法一致,只是试样无需预先干燥。每毫升lmol/L氢氧化钠溶液相当于本品(C10H12O3)180.2mg。
毒性LD50值为7.17g/kg(小鼠经口摄入)。
化学性质无色小晶体或白色结晶粉末,无特殊气味。难溶于水(25℃时溶解度约为0.088g/100ml),易溶于乙醇、乙醚、丙酮和冰醋酸等有机溶剂中。
用途 生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对羟基苯甲酸甲酯 methyl 4-hydroxybenzoate 99-76-3 C8H8O3 152.15 对羟基苯甲酸 4-hydroxy-benzoic acid 99-96-7 C7H6O3 138.123 —— isopropyl 4-hydroxy-3-iodobenzoate 37470-70-5 C10H11IO3 306.1 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Isopropyl 4-acetoxybenzoate 27739-15-7 C12H14O4 222.241 乙基4-乙酰氧基苯甲酸酯 ethyl 4-acetoxybenzoate 13031-45-3 C11H12O4 208.214 对羟基苯甲酸 4-hydroxy-benzoic acid 99-96-7 C7H6O3 138.123 4-乙酰氧基苯甲酸丙酯 n-propyl 4-acetoxybenzoate 27739-13-5 C12H14O4 222.241 —— 4-Acetoxybenzoic acid butyl ester 27739-14-6 C13H16O4 236.268 —— 2-Methylpropyl 4-acetyloxybenzoate 27801-52-1 C13H16O4 236.268 4-乙酰氧基苯甲酸甲酯 methyl 4-acetoxybenzoate 24262-66-6 C10H10O4 194.187 4-异丙氧苯甲酸 4-isopropoxybenzoic acid 13205-46-4 C10H12O3 180.203 —— Benzyl-p-acetoxy-benzoat 54835-09-5 C16H14O4 270.285
反应信息
-
作为反应物:参考文献:名称:新的丁苯芬唑胺的N-草酰衍生物的合成和杀虫性能评估。摘要:合成了一系列在芳基上含有羧酸或酯取代基的新型N'-叔丁基-N'-3,5-二甲基苯甲酰基-N-芳氧基草酰-N-4-乙基苯甲酰肼,并对其杀幼虫活性进行了评估。生物测定的结果表明,这些标题化合物中的一些具有比RH5849(N-叔丁基-N,N'-二苯甲酰肼)更高的杀幼虫活性,但与母体化合物(丁苯脲类)相比,效果不佳。芳基上的羧酸取代基对于高杀幼虫活性是必不可少的。与母体化合物相比,这些衍生物表现出不同的物理性质,例如,在有机溶剂中的溶解度更高。毒性试验表明,这些衍生物可能诱发过早的,异常的和致命的幼虫蜕皮。DOI:10.1021/jf048834e
-
作为产物:参考文献:名称:使用 Houben-Hoesch 反应从富含同位素的酚中合成 13C 标记的对羟基苯甲酸酯摘要:对羟基苯甲酸酯是多种消费品中的抗菌添加剂。然而,在废水消毒过程中由对羟基苯甲酸酯形成的卤代化合物是一个潜在的环境问题。为了识别这些转化产物并研究它们的形成机制,开发了一种在苯环内特定位置用稳定同位素碳 13 标记对羟基苯甲酸乙酯的合成路线。这种有效的两步法从市售的13 C 标记的苯酚开始,包括 (1) 通过与三氯乙腈的 Houben-Hoesch 反应对苯酚进行初始酰化,然后 (2) 对所得三氯甲基酮进行改进的卤仿反应,得到对应13C 标记的对羟基苯甲酸乙酯的总产率为 65%–80%。还研究了改进的卤仿反应的范围,允许合成衍生自伯醇或仲醇的其他对羟基苯甲酸酯,包括13 C-和氘标记的酯。此外,4-羟基苯甲酸可以直接由常见的三氯甲基酮中间体在用氢氧化锂处理后形成。该方案补充了现有的制备13 C 标记的对羟基苯甲酸酯衍生物的方法,并提供了在 Houben-Hoesch 反应(形成对二取代产物)DOI:10.1002/jlcr.3992
文献信息
-
Catalytic Asymmetric Synthesis of a Tertiary Benzylic Carbon Center via Phenol-Directed Alkene Hydrogenation作者:Seb Caille、Rich Crockett、Krishnakumar Ranganathan、Xiang Wang、Jacqueline C. S. Woo、Shawn D. WalkerDOI:10.1021/jo200941r日期:2011.7.1required for this transformation is described. To complete the process, a highly enantioselective hydrogenation step afforded the target (1). The importance of the phenol group to the success of this asymmetric transformation is discussed.
-
Supported Catalytically Active Supramolecular Hydrogels for Continuous Flow Chemistry作者:Jennifer Rodon Fores、Miryam Criado‐Gonzalez、Alain Chaumont、Alain Carvalho、Christian Blanck、Marc Schmutz、Christophe A. Serra、F. Boulmedais、Pierre Schaaf、Loïc JierryDOI:10.1002/anie.201909424日期:2019.12.19walls of a supporting porous material. We applied this strategy to grow an esterase-like catalytically active supramolecular hydrogel (CASH) in an open-cell polymer foam, filling the whole interior space. Our supported CASH material is highly efficient towards inactivated esters and enables the kinetic resolution of racemates. This hybrid material is robust enough to be used in continuous flow reactors
-
[EN] NOVEL ANTIMICROBIAL PEPTIDES AND THEIR APPLICATION<br/>[FR] NOUVEAUX PEPTIDES ANTIMICROBIENS ET LEUR APPLICATION申请人:INFINITEC ACTIVOS S L公开号:WO2015135896A1公开(公告)日:2015-09-17The present invention relates to a compound of formula (I) and to compositions comprising said compound, as well as the use of said compound and/or said compositions to prevent fungal and/or bacterial growth.本发明涉及式(I)的化合物,以及包含该化合物的组合物,以及使用该化合物和/或所述组合物来防止真菌和/或细菌生长。
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
RECORDING MATERIAL PRODUCED USING NON-PHENOL COMPOUND申请人:NIPPON SODA CO., LTD.公开号:US20150284321A1公开(公告)日:2015-10-08An object of the present invention is to provide a recording material or a recording sheet using, as a color-developing agent, a non-phenol compound that is a safe compound in no danger of corresponding to an endocrine disruptor and is good in color developing performance. The non-phenol compound used in the present invention is at least one selected from the group consisting of compounds represented by the following formulas (I) to (III).
表征谱图
-
氢谱1HNMR
-
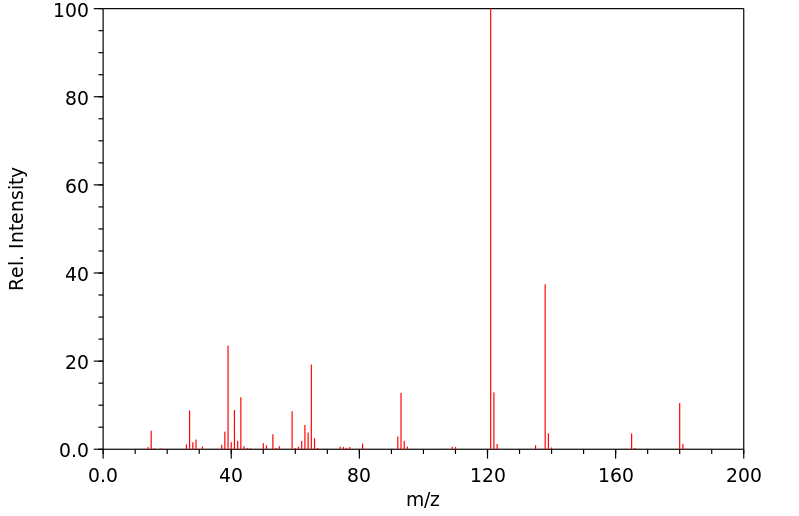
质谱MS
-
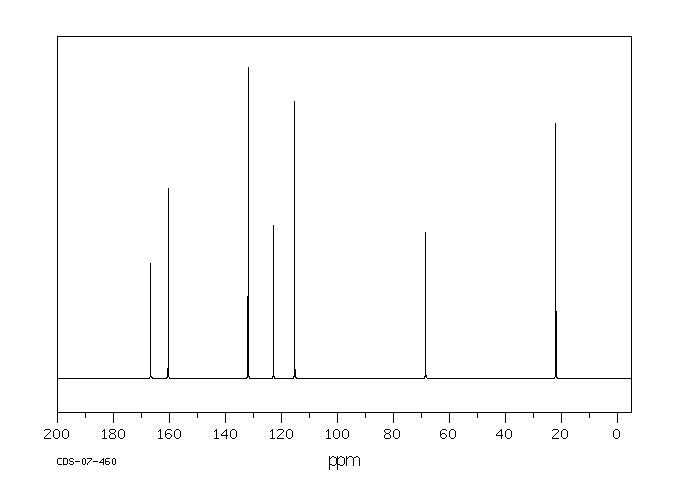
碳谱13CNMR
-
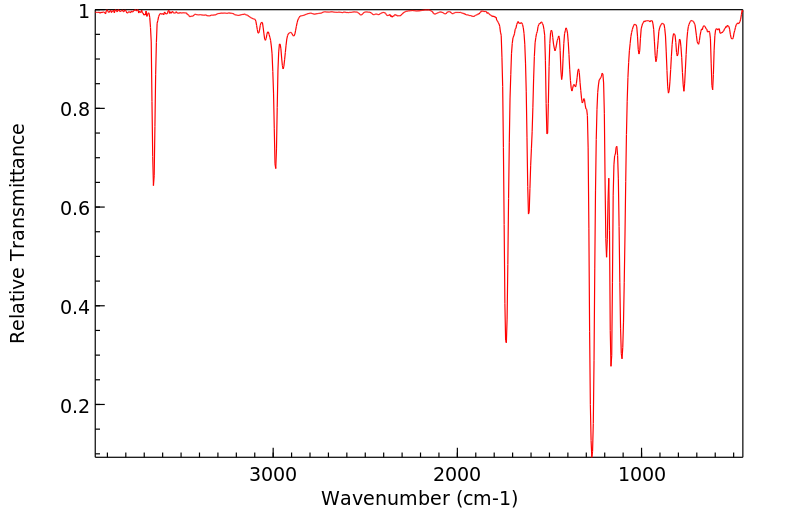
红外IR
-
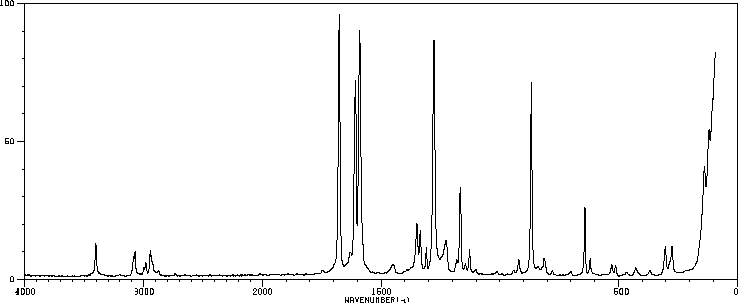
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫