毒理性
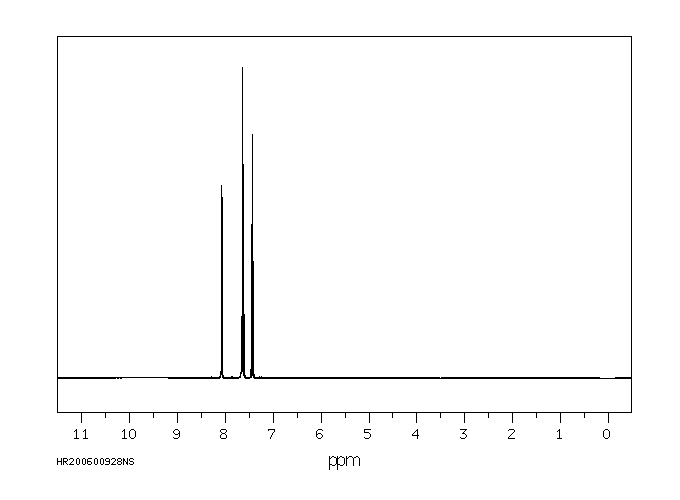
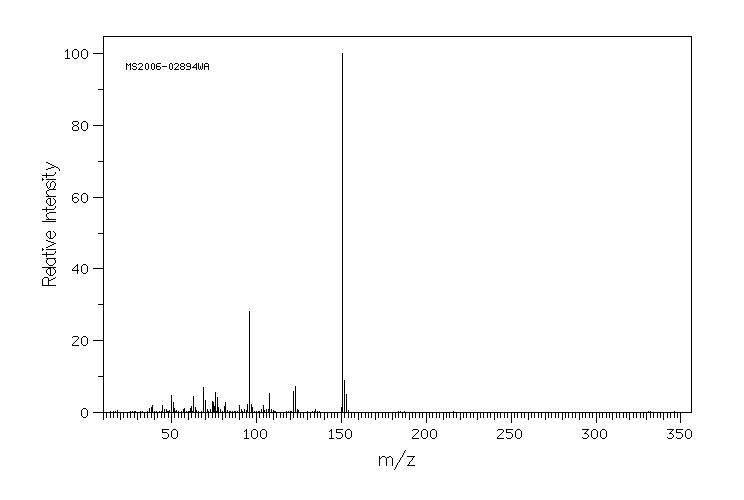
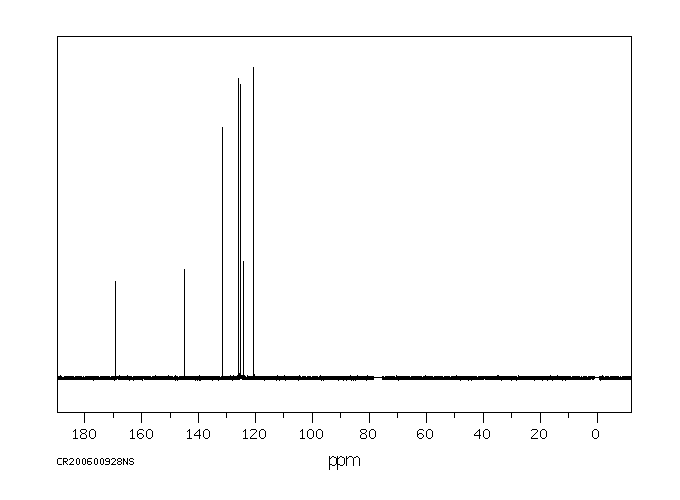
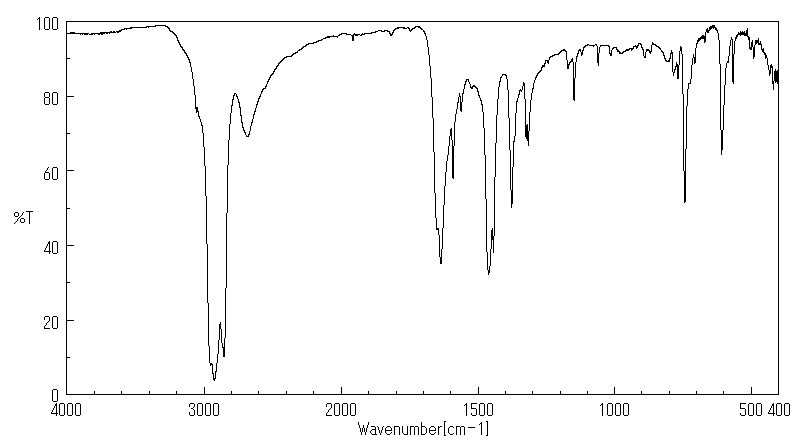
识别和使用:1,2-苯并异噻唑啉-3-酮(BIT)是一种灰白色至浅黄色的固体。它在美囀作为农药使用进行了注册,但批准的农药用途可能会定期更改,因此必须咨询联邦、州和地方当局以获取当前批准的用途。BIT在化妆品中用作抗菌剂;在制造一次性无粉聚氯乙烯手套时用作杀藻剂;在工业中广泛用作基于水的溶液,如糊剂、涂料和切削油的防腐剂。它还用于私人和公共卫生区域的消毒剂和其他杀生物产品,罐内防腐剂,薄膜防腐剂,纤维、皮革、橡胶和聚合材料的防腐剂,砖石防腐剂,液体冷却和处理系统的防腐剂,金属加工液体的防腐剂,以及防腐剂和尸体防腐剂。人类暴露和毒性:在足够剂量和持续时间的皮肤暴露下,BIT可能导致易感人群的皮肤致敏和过敏性接触皮炎。在一篇报告中,一名26岁的男性在一家生产洗涤剂的化工厂工作期间,通过吸入BIT引起了职业性哮喘和鼻炎。动物研究:严重眼睛刺激物。亚慢性口服毒性研究显示,在重复口服给药后出现系统性影响,包括大鼠体重减轻、胃前壁增生发生率增加和非腺胃病变。在狗中,这些影响发生在比大鼠更低的剂量下,包括血液化学变化(血浆白蛋白、总蛋白和丙氨酸转氨酶降低)和绝对肝重量增加。在大鼠中进行了发育毒性研究,母体影响包括体重增加减少、食物消耗减少和临床毒性迹象(可听到呼吸声、肛门生殖区毛发染色、鼻区周围干燥的棕色物质)以及死亡率增加。发育影响包括骨骼异常增加(颅骨骨骼的额外骨化位点、未骨化的胸骨),但没有外部或内脏异常。
IDENTIFICATION AND USE: 1,2-Benzisothiazoline-3-one (BIT) is an off-white to yellowish solid. It is registered for pesticide use in the USA but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. BIT is used as an antimicrobial agent in cosmetics; used as a slimicide in the manufacture of disposable powder-free polyvinyl chloride gloves; widely used in industry as a preservative in water-based solutions such as pastes, paints and cutting oils. It is also used in private area and public health area disinfectants and other biocidal products, in-can preservatives, film preservatives fibre, leather, rubber and polymerized materials preservatives, masonry preservatives, preservatives for liquid-cooling and processing systems, metalworking-fluid preservatives, embalming and taxidermist fluids. HUMAN EXPOSURE AND TOXICITY: Dermal exposure to BIT at sufficient dose and duration can produce skin sensitization and allergic contact dermatitis in susceptible humans. Occupational asthma and rhinitis caused by inhalation of BIT was reported in a 26-year-old man employed in a chemical factory producing detergents. ANIMAL STUDIES: Severe eye irritant. Subchronic oral toxicity studies showed systemic effects after repeated oral administration including decreased body weight, increased incidence of forestomach hyperplasia, and non-glandular stomach lesions in rats. In dogs, the effects occurred at lower doses than in rats, and included alterations in blood chemistry (decreased plasma albumin, total protein, and alanine aminotransferase) and increased absolute liver weight. Developmental toxicity studies were conducted in rats with maternal effects including decreased body weight gain, decreased food consumption, and clinical toxicity signs (audible breathing, haircoat staining of the anogenital region, dry brown material around the nasal area) as well as increased mortality. Developmental effects consisted of increases in skeletal abnormalities (extra sites of ossification of skull bones, unossified sternebra) but not external or visceral abnormalities.
来源:Hazardous Substances Data Bank (HSDB)