毒扁豆碱 | 57-47-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:102-104 °C(lit.)
-
比旋光度:D17 -76° (c = 1.3 in chloroform); D25 -120° (benzene)
-
沸点:418.29°C (rough estimate)
-
密度:1.166±0.06 g/cm3 (20 ºC 760 Torr)
-
闪点:>100℃
-
溶解度:氯仿(微溶、超声处理)、DMSO(微溶)、乙醇(微溶)、甲醇
-
物理描述:Physostigmine is a white, odorless, microcrystalline powder. Used as a cholinergic (anticholinesterase) agent and as a veterinary medication. (EPA, 1998)
-
颜色/状态:ORTHORHOMBIC SPHENOIDAL PRISMS OR CLUSTERS OF LEAFLETS FROM ETHER OR BENZENE
-
气味:ODORLESS
-
稳定性/保质期:
SOLID & SOLNS TURN RED ON EXPOSURE TO HEAT, LIGHT, AIR, & ON CONTACT WITH TRACES OF METALS.
-
旋光度:SPECIFIC OPTICAL ROTATION @ 17 °C/D= -76 DEG (CONCENTRATION BY VOLUME = 1.3 GIN 100 ML CHLOROFORM); @ 25 °C/D= -120 DEG (BENZENE)
-
分解:When heated to decomposition it emits toxic fumes of oxides of nitrogen.
-
解离常数:K1= 7.6X10-7; K2= 5.7X10-13
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:20
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.53
-
拓扑面积:44.8
-
氢给体数:1
-
氢受体数:4
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1(a)
-
危险品标志:T+
-
安全说明:S23,S25,S45
-
危险类别码:R26/28
-
WGK Germany:3
-
危险品运输编号:UN 1544 6.1/PG 1
-
RTECS号:TJ2100000
-
包装等级:II
-
危险类别:6.1(a)
SDS
制备方法与用途
毒扁豆碱亦称“依色林”,是一种吲哚类生物碱,化学式为C15H21N3O2。它存在于非洲西部的一种豆科植物——毒扁豆的种子中,含量约为0.1%。在乙醚中析出时呈片状结晶,熔点为105~106°C,该晶体不稳定,易变为熔点86~87°C的结晶。微溶于水,可溶于乙醇、苯、氯仿或脂肪油中。毒扁豆碱能抑制胆碱酯酶而引起机体中毒,是一种副交感神经兴奋剂,其中毒症状类似于神经性毒剂。口服中毒的人致死剂量为6~10毫克/人。
生物活性Physostigmine(Eserine)是一种可逆的乙酰胆碱酯酶(AChE)抑制剂,能够透过血脑屏障并刺激中枢胆碱能神经传递。它能够逆转阿尔茨海默病转基因小鼠的记忆缺陷,并且也是抗胆碱能中毒的解毒剂。
体内研究- 改善记忆功能:在异氟烷麻醉下,Physostigmine(0.1, 0.2 mg/kg)剂量大于等于0.2 mg/kg时可以延迟雄性Sprague-Dawley大鼠从麻醉中苏醒的时间。
- 转基因小鼠实验:Physostigmine (Eserine; 0.03-0.3 mg/kg;s.c.; 每日一次,持续6周) 能够在一定程度上改善heterozygous transgenic mice(Tg(+))的环境和提示记忆缺陷,使其更接近于非转基因小鼠的记忆表现。
毒扁豆碱为无色片状结晶,无特殊气味。遇光、热及微量金属时逐渐变红。该物质在碱性条件下不稳定,水解后生成毒扁豆酚碱,并进一步氧化生成红色的依色林红。其熔点为105-106°C,比旋光度[α]17D -76° (1.3%,氯仿),[α]25D -120°(苯)。易溶于二氯甲烷,可溶于氯仿(1:1)、乙醇(1:10)和乙醚(1:30),微溶于水。毒扁豆碱有毒性,成年鼠口服LD50为4.5毫克/公斤,小鼠为3毫克/公斤。
用途毒扁豆碱主要用于生化研究、药物合成中间体以及抗胆碱酯酶药的制备。它能够抑制体内胆碱酯酶的活力,是一种副交感神经兴奋剂,主要用于治疗青光眼,其效力较毛果芸香碱强而持久,可维持几个小时至几天。目前在中国还被用作中药麻醉的催醒药物。
生产方法毒扁豆碱存在于豆科植物——毒扁豆(Physostigma venenosum)的种子中。通过乙醇或丙酮萃取出生物碱后,将醇溶液或丙酮溶液与双氧水作用,在低温下蒸发未反应的生物碱,再用醚溶解生成毒扁豆碱N-氧化物。使用乙酸和锌粉还原可以恢复为毒扁豆碱。
安全信息 性质有毒物质
毒性分级剧毒
急性毒性大鼠口服LD50:4.5毫克/公斤;小鼠口服LD50: 3毫克/公斤
可燃性危险特性可燃,火场分解产生有毒氮氧化物气体
储运特性库房低温通风干燥;与食品原料分开存放
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (3AS,8AR)-5-甲氧基-1,3A,8-三甲基-1,2,3,3A,8,8A-六氢吡咯并[2,3-B]吲哚 (-)-esermethole 65166-97-4 C14H20N2O 232.326 —— (-)-N1-norphysostigmine 116103-18-5 C14H19N3O2 261.324 —— O-benzyleseroline 141613-05-0 C20H24N2O 308.423 氧化毒扁豆碱 (+/-)-eseroline 469-22-7 C13H18N2O 218.299 —— eseroline 408340-83-0 C13H18N2O 218.299 —— 5-methoxy-8-methyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indole —— C13H18N2O 218.299 —— N8-norphysostigmine 19573-10-5 C14H19N3O2 261.324 [(3aR,8bS)-3,4,8b-三甲基-2,3a-二氢-1H-吡咯并[2,3-b]吲哚-7-基] 3,4-二氢-1H-异喹啉-2-羧酸酯 (3aS,cis)-1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo[2,3-b]indol-5-yl-3,4-dihydro-2(1H)-isoquinolinecarboxylate 139314-01-5 C23H27N3O2 377.486 —— 5-(benzyloxy)-1,3a-dimethyl-8-formyl-3,3a,8,8a-tetrahydropyrrolo<2,3-b>indol-2-one —— C20H20N2O3 336.39 —— 5-methoxy-1,3-dimethyl-3-(2-methylamino-ethyl)-indolin-2-one 143947-74-4 C14H20N2O2 248.325 —— (3S)-(5-methoxy-1,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-3-yl)acetaldehyde 153109-52-5 C13H15NO3 233.267 —— (5-methoxy-1,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-3-yl)acetonitrile 2291-51-2 C13H14N2O2 230.266 —— 1,3-dimethyl-3-cyanomethyl-5-tetrahydropyranyloxyoxindole 161986-97-6 C17H20N2O3 300.357 —— 5-(benzyloxy)-8-formyl-3a-methyl-3,3a,8,8a-tetrahydro-2H-furo<2,3-b>indol-2-one 141613-03-8 C19H17NO4 323.348 —— 5-(benzyloxy)-8-(tert-butoxycarbonyl)-3a-methyl-3,3a,8,8a-tetrahydro-2H-furo<2,3-b>indol-2-one 141613-01-6 C23H25NO5 395.455 —— (3S)-5-methoxy-1,3-dimethyl-3-[(E)-2-tri(propan-2-yl)silyloxyethenyl]indol-2-one 153109-54-7 C22H35NO3Si 389.61 —— 1,3-dimethyl-5-tetrahydropyranyloxyoxindole 161467-02-3 C15H19NO3 261.321 1,3-二甲基-5-甲氧基吲哚啉-2-酮 1,3-dimethyl-5-methoxyindolin-2-one 116707-99-4 C11H13NO2 191.23 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 依斯的明 eptastigmine 101246-68-8 C21H33N3O2 359.512 —— (+/-)-esermethole 2731-01-3 C14H20N2O 232.326 —— 5-ethoxy-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro-pyrrolo[2,3-b]indole 33066-67-0 C15H22N2O 246.352 —— [(3aR,8bS)-4-formyl-3,8b-dimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-methylcarbamate 128562-54-9 C15H19N3O3 289.334 氧化毒扁豆碱 (+/-)-eseroline 469-22-7 C13H18N2O 218.299 —— (-)-norphysostigmine 19573-10-5 C14H19N3O2 261.324 —— (3aS-cis)-1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo-[2,3-b]indol-5-yl 3,4-dihydro-2(1H)-isoquinolinecarboxylate —— C23H27N3O2 377.486 [(3aR,8bS)-3,4,8b-三甲基-2,3a-二氢-1H-吡咯并[2,3-b]吲哚-7-基] 3,4-二氢-1H-异喹啉-2-羧酸酯 (3aS,cis)-1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo[2,3-b]indol-5-yl-3,4-dihydro-2(1H)-isoquinolinecarboxylate 139314-01-5 C23H27N3O2 377.486 —— 1-benzyl-1-demethylesermethole —— C20H24N2O 308.423
反应信息
-
作为反应物:参考文献:名称:Total Syntheses and Anticholinesterase Activities of (3aS)-N(8)-Norphysostigmine, (3aS)-N(8)-Norphenserine, Their Antipodal Isomers, and Other N(8)-Substituted Analogues摘要:N(8)-Benzylesermethole (6) was prepared from 5-methoxytryptamine (1) in five steps. Resolution of compound 6 by dibenzoyl- and ditoluyltartaric acid provided enantiomers (-)- and (+)-7. After demethylation, reaction with isocyanates and catalytic debenzylation over hydrogen, the total syntheses of(-)-and (+)-N(8)-norphysostigmine [(-)-and (+)-11] and (-)-and (+)N(8)-norphenserine [(-)-and (+)-12] were accomplished. (-)-N(8)-Norphysostigmine [(-)-11] and (-)-N(8)-norphenserine [(-)-12] were also obtained by transformations of natural physostigmine [(-)-13] and phenserine [(-)-14] prepared from(-)-13. The absolute configurations and optical purity of compounds (-)-11, (-)-12, (+)-11, and (+)-12 were confirmed by a comparison of their optical rotations with those of the compounds synthesized from physostigmine [(-)-13]. The anticholinesterase activities of N(8)-nor-and N(8)-substituted analogues, (-)-and (+)-9, -10, -11, -12, 15, and 16, were compared with those of physostigmine [(-)-and (+)-13] and phenserine [(-)- and (+)-14] and are reported.DOI:10.1021/jm970210v
-
作为产物:参考文献:名称:The enantioselective synthesis of (-)-physostigmine via chiral sulfoxides摘要:The total synthesis of naturally occurring (-)-physostigmine is described. The key element for the asymmetric induction is the chirality transfer from optically active 2-(alkylsulfinyl)indoles to indoline butyrolactones bearing two chiral centers. Novel features of this synthesis involve the use of a new class of sulfoxylating agents, N-(alkylsulfinyl)oxazolidinones, to prepare the starting indolyl sulfoxides and the correlation of the size of the alkyl group on the sulfoxide with the degree of asymmetric induction. The overall synthesis requires a dozen steps from commercially available 5-(benzyloxy)indole.DOI:10.1021/ja00040a013
文献信息
-
NOVEL GLUCOKINASE ACTIVATORS AND METHODS OF USING SAME申请人:Ryono Denis E.公开号:US20080009465A1公开(公告)日:2008-01-10Compounds are provided which are phosphonate and phosphinate activators and thus are useful in treating diabetes and related diseases and have the structure wherein is a heteroaryl ring; R 4 is —(CH 2 ) n -Z-(CH 2 ) m —PO(OR 7 )(OR 8 ), —(CH 2 ) n Z-(CH 2 ) m —PO(OR 7 )R g , —(CH 2 ) n -Z-(CH 2 ) m —OPO(OR 7 )R g , —(CH 2 ) n Z—(CH 2 ) m —OPO(R 9 )(R 10 ), or —(CH 2 ) n Z—(CH 2 ) m —PO(R 9 )(R 10 ); R 5 and R 6 are independently selected from H, alkyl and halogen; Y is R 7 (CH 2 ) s or is absent; and X, n, Z, m, R 4 , R 5 , R 6 , R 7 , and s are as defined herein; or a pharmaceutically acceptable salt thereof. A method for treating diabetes and related diseases employing the above compounds is also provided.提供了磷酸酯和磷酸酯激活剂,因此在治疗糖尿病和相关疾病方面非常有用,并具有以下结构: 其中 是杂环芳基环; R 4 为—(CH 2 ) n -Z-(CH 2 ) m —PO(OR 7 )(OR 8 )、—(CH 2 ) n Z-(CH 2 ) m —PO(OR 7 )R g 、—(CH 2 ) n -Z-(CH 2 ) m —OPO(OR 7 )R g 、—(CH 2 ) n Z—(CH 2 ) m —OPO(R 9 )(R 10) 或—(CH 2 ) n Z—(CH 2 ) m —PO(R 9 )(R 10) ; R 5 和R 6 分别选择自H、烷基和卤素; Y为R 7 (CH 2 ) s 或不存在;以及 X、n、Z、m、R 4 、R 5 、R 6 、R 7 和s如本文所定义;或其药用盐。 还提供了一种利用上述化合物治疗糖尿病和相关疾病的方法。
-
Novel Compounds申请人:Chhipa Laxmikant公开号:US20100168110A1公开(公告)日:2010-07-01The present invention discloses a novel thyroid like compounds of formula (I), wherein R 1 R 2 , R 3 , R 4 and Z are as defined in the specification, method for its preparation, composition containing such compounds and use of such compounds and composition as medicament. Further, compounds of formula (I) has significantly low binding affinity to thyroid receptors and thus considerably devoid of thyrotoxic effects. The invention also relates to the use of the compound of formula (I) for the preparation of a medicament for treating various disease conditions such as obesity, dyslipidemia, metabolic syndrome and co-morbidities associated with metabolic syndrome.
-
[EN] BIS-HETEROARYL DERIVATIVES AS MODULATORS OF PROTEIN AGGREGATION<br/>[FR] DÉRIVÉS BIS-HÉTÉROARYLIQUES EN TANT QUE MODULATEURS DE L'AGRÉGATION DES PROTÉINES申请人:NEUROPORE THERAPIES INC公开号:WO2017020010A1公开(公告)日:2017-02-02The present invention relates to certain bis-heteroaryl compounds, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto- temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
-
Amino-substituted heterocycles, compositions thereof, and methods of treatment therewith申请人:D'Sidocky Neil R.公开号:US20080242694A1公开(公告)日:2008-10-02Provided herein are Heterocyclic Compounds having the following structure: wherein R 1 , R 2 , X, Y and Z are as defined herein, compositions comprising an effective amount of a Heterocyclic Compound and methods for treating or preventing cancer, inflammatory conditions, immunological conditions, metabolic conditions and conditions treatable or preventable by inhibition of a kinase pathway comprising administering an effective amount of a Heterocyclic Compound to a patient in need thereof.
-
[EN] ALKOXY BIS-HETEROARYL DERIVATIVES AS MODULATORS OF PROTEIN AGGREGATION<br/>[FR] DÉRIVÉS BIS-HÉTÉROARYLIQUES D'ALCOXY UTILISÉS EN TANT QUE MODULATEURS DE L'AGRÉGATION DE PROTÉINES申请人:UCB BIOPHARMA SPRL公开号:WO2018138085A1公开(公告)日:2018-08-02The present invention relates to certain bis-heteroaryl compounds, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto-temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
表征谱图
-
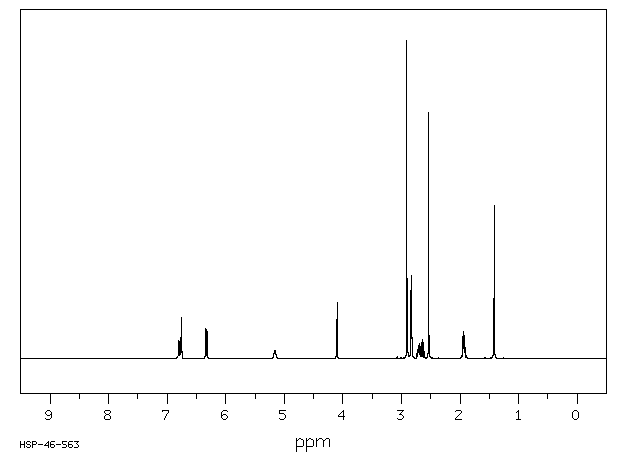
氢谱1HNMR
-
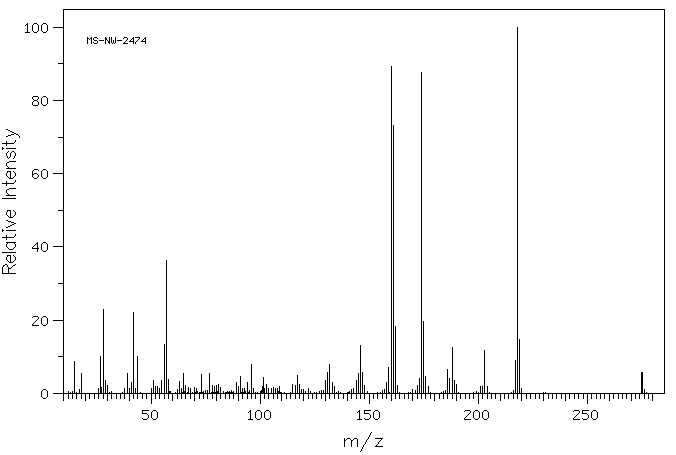
质谱MS
-
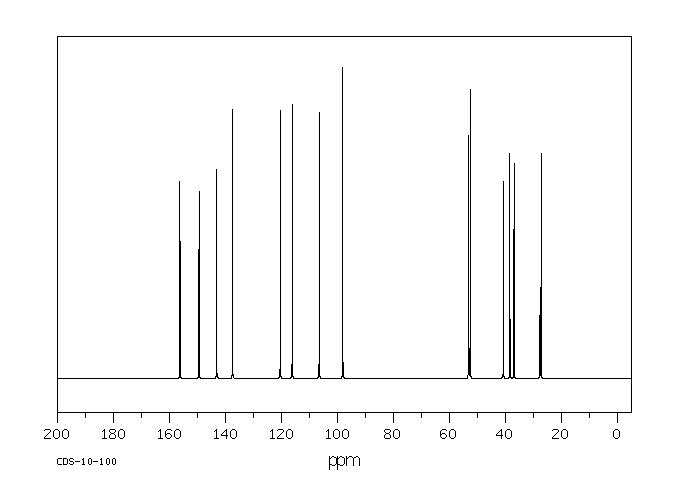
碳谱13CNMR
-
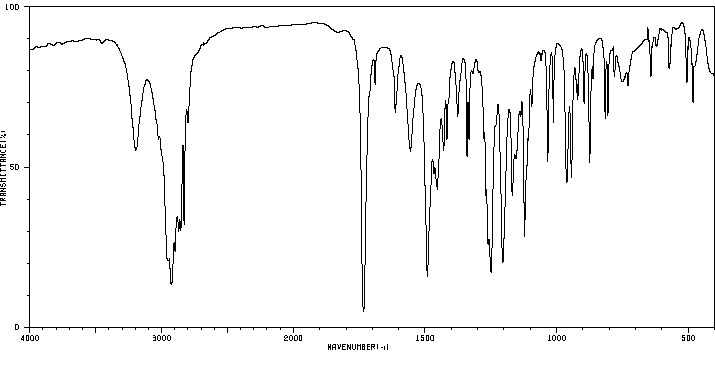
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息