硅镁型吸附剂 | 1343-88-0
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:1910°C
-
密度:3.21
-
溶解度:Insoluble
-
稳定性/保质期:
如果按照规格使用和储存,则不会发生分解,并且没有已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):-1.12
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:3
-
氢给体数:0
-
氢受体数:3
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2839900090,28399000
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:VV7340000
SDS
| Name: | Florisil Material Safety Data Sheet |
| Synonym: | Magnesium Silicat |
| CAS: | 1343-88-0 |
Synonym:Magnesium Silicat
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1343-88-0 | Magnesium Silicate | 100.0 | 215-681-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
No information regarding eye irritation and other potential effects was found.
Skin:
No information regarding skin irritation and other potential effects was found.
Ingestion:
The toxicological properties of this substance have not been fully investigated.
Inhalation:
The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Gently lift eyelids and flush continuously with water.
Skin:
Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water fog, dry chemical, carbon dioxide, or regular foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Avoid contact with skin and eyes.
Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Store in a cool, dry place.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1343-88-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble in water.
Specific Gravity/Density:
Molecular Formula:
Molecular Weight:
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable.
Conditions to Avoid:
None reported.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
None.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1343-88-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Magnesium Silicate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 1343-88-0: 0
Canada
CAS# 1343-88-0 is listed on Canada's DSL List.
CAS# 1343-88-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1343-88-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
取试样500mg与10ml稀盐酸试液(TS-117)混合,过滤后用氨试液(TS-13)中和至石蕊试纸呈中性。中和后的滤液进行镁试验(IT-21),结果呈阳性。
再取少量磷酸铵钠结晶于无色火焰上熔化成小球,并趁热蘸取样品,再次熔融后二氧化硅会浮于小球表面,放冷后形成网状结构的不透明小球。这表明硅试验呈阳性。
溶解性
该物质不溶于水和乙醇(OT-42)。遇无机酸易分解,10%浆状体的pH值为7.0~10.8。
准确称取试样约1.5g,放入250ml锥形烧瓶中,加入1mol/L硫酸50.0ml,在蒸汽浴上煮解1h。冷却至室温后,加甲基橙试液(TS-148)1滴,并用1mol/L氢氧化钠液滴定过量的酸。每毫升1mol/L硫酸相当于MgO 0.15mg。
二氧化硅含量的分析称取约700mg试样(精确至0.1mg),放入150ml烧杯中,加入20ml 1mol/L硫酸,并于蒸汽浴上加热1.5h。滗去上层清液,用无灰滤纸过滤残余物,并用水分3次洗涤。将残渣移入铂坩埚内,在干后并灼烧30min,冷却、称重。加入氢氟酸6ml和硫酸3滴,蒸发至干后再灼烧5min,冷却后再次称重。试样失去的重量即为SiO2的重量。
毒性FAO/WHO(2001)未对ADI值作特殊规定。
GRAS(FDA,§182.2437,2000)。
使用限量FAO/WHO (1984, g/kg):粉状乳制品(奶粉、奶油粉、高脂奶粉、半奶油粉) 10(单独或与其他抗结剂合用量,仅适用于自动售货机);涂层用糖粉和葡萄糖粉 15(不得有淀粉存在)。
化学性质合成的硅酸镁,氧化镁(MgO)与二氧化硅(SiO2)的摩尔比平均为2:5。白色细粉,无臭、无味、无沙砾感,略有吸湿性。易受无机酸分解,10%混悬液的pH值为7.0~10.8,不溶于水和乙醇。
用途抗结剂;助滤剂;被膜剂;糖果抛光涂釉剂;胶姆糖扑粉剂;大米涂层剂;(药品)制酸剂。根据GB 2760-90列为加工助剂,FDA规定可用作餐桌用盐,限量2%。
其他用途用于有机氯残留分析。
用于聚醚吸附剂,具有吸附和脱色的作用。
用于柱层析,杀虫剂分析。
反应信息
-
作为反应物:参考文献:名称:Use of terpene alcohol ethoxylates as solubilizers in cosmetic or pharmaceutical preparations or concentrates for food preparations摘要:使用十碳原子的萜烯醇,经过3至10摩尔的乙氧基化处理后,作为化妆品或药物制剂中难溶于水或难分散于水的化合物的溶解剂,或者作为食品制剂浓缩物的溶解剂,用量至少为制剂成品重量的3%或浓缩物重量的3%。公开号:US20020076426A1
-
作为试剂:描述:丁烯酮 、 1-叔丁氧羰基哌啶-4-甲醛 在 四氢吡咯 、 溶剂黄146 、 三甲胺 、 柠檬酸 、 硅镁型吸附剂 、 N-溴代丁二酰亚胺(NBS) 、 disodium;dioxido-oxo-sulfanylidene-λ6-sulfane 、 potassium tert-butylate 、 盐酸 、 N,N'-羰基二咪唑 、 N,N-二异丙基乙胺 、 potassium hydrogensulfate 、 对甲苯磺酸 、 水 作用下, 以 环己烷 、 水 、 N,N-二甲基甲酰胺 、 甲苯 、 二氯甲烷 、 甲醇 、 2-甲基四氢呋喃 、 四氢呋喃 、 乙酸乙酯 、 正庚烷 、 乙腈 为溶剂, 以65 %的产率得到4-(4-(1-isopropyl-7-oxo-1,4,6,7-tetrahydrospiro[indazole-5,4'-piperidine]-1'-carbonyl)-6-methoxypyridin-2-yl)benzoic acid参考文献:名称:[EN] COMBINATIONS OF DIACYLGLYCEROL ACYLTRANSFERASE 2 INHIBITORS AND ACETYL-COA CARBOXYLASE INHIBITOR
[FR] COMBINAISONS D'INHIBITEURS DE DIACYLGLYCÉROL ACYLTRANSFÉRASE 2 ET D'INHIBITEUR D'ACÉTYL-COA CARBOXYLASE摘要:本文描述了包含2-{5-[(3-乙氧基吡啶-2-基)氧基]吡啶-3-基}-N-[(3S,5S)-5-氟哌啶-3-基]嘧啶-5-羧酰胺或其药用可接受盐的药物组合物,用于治疗肝病及相关疾病。还描述了包含2-{5-[(3-乙氧基吡啶-2-基)氧基]吡啶-3-基}-N-[(3S,5S)-5-氟哌啶-3-基]嘧啶-5-5羧酰胺或其药用可接受盐以及4-(4-(1-异丙基-7-氧代-1,4,6,7-四氢螺[吲唑-5,4'-哌啶]-1'-羰基)-6-甲氧基吡啶-2-基)苯甲酸或其药用可接受盐的组合物,用于治疗肝病及相关疾病。公开号:WO2021171163A1
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
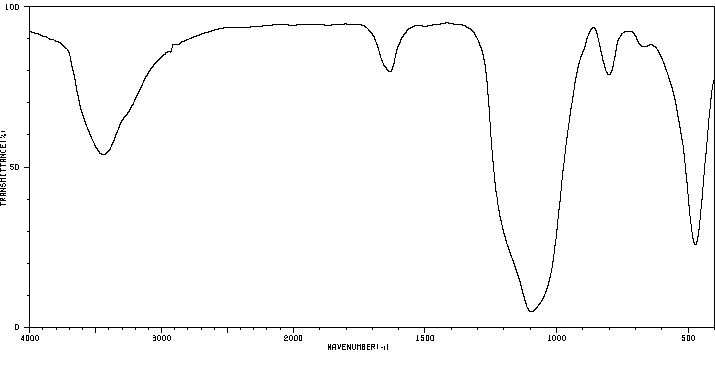
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息