3-乙基-3-己醇 | 597-76-2
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-61.15°C (estimate)
-
沸点:160.1°C
-
密度:0.83
-
LogP:2.622 (est)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
安全说明:S26,S36/37/39
-
储存条件:存储条件:2-8°C,密封,干燥。
SDS
制备方法与用途
生产方法简介:
-
羰基合成法:以丙烯和合成气为原料,经羰基合成反应生成丁醛;两分子丁醛缩合脱水得2-乙基(-2-)己烯醛,再加氢得2-乙基(-1-)己醇。
-
乙醛缩合法:以乙醛缩合成丁醇醛,脱水得巴豆醛;加氢得正丁醛,然后将两分子正丁醛缩合脱水得2-乙基(-2-)己烯醛,再加氢得2-乙基(-1-)己醇。
生产方法简介:
-
羰基合成法:以丙烯和合成气为原料,经羰基合成反应生成丁醛;两分子丁醛缩合脱水得2-乙基(-2-)己烯醛,再加氢得2-乙基(-1-)己醇。
-
乙醛缩合法:以乙醛缩合成丁醇醛,脱水得巴豆醛;加氢得正丁醛,然后将两分子正丁醛缩合脱水得2-乙基(-2-)己烯醛,再加氢得2-乙基(-1-)己醇。
2-乙基已醇在增塑剂领域习惯称为辛醇,它是重要的化工原料。国外生产的辛醇除用于生产增塑剂外,还用来生产丙烯酸辛酯或作为表面活性剂等,主要可用来生产:苯二甲酸二辛酯(DOP)、乙二酸二辛酯(DOA)、偏苯三酸三辛酯(TOTM)、其他增塑剂、丙烯酸辛酯、表面活性剂类、润骨油添加剂、采矿用、柴油机燃料添加剂、溶剂和配药、防锈剂酯和其他化学品。辛醇本身是有用的溶剂、消沫剂、分散剂、润滑剂,其系列产品主要是邻苯二甲酸二辛酯等增塑剂和丙烯酸辛酯等。以辛醇为原料生产的邻苯二甲酸二辛酯是PVC的主要增塑剂,用于PVC的消耗量约占其总消费量的95%。
用途2-乙基已醇在增塑剂领域习惯称为辛醇,它是重要的化工原料。国外生产的辛醇除用于生产增塑剂外,还用来生产丙烯酸辛酯或作为表面活性剂等,主要可用来生产:苯二甲酸二辛酯(DOP)、乙二酸二辛酯(DOA)、偏苯三酸三辛酯(TOTM)、其他增塑剂、丙烯酸辛酯、表面活性剂类、润骨油添加剂、采矿用、柴油机燃料添加剂、溶剂和配药、防锈剂酯和其他化学品。辛醇本身是有用的溶剂、消沫剂、分散剂、润滑剂,其系列产品主要是邻苯二甲酸二辛酯等增塑剂和丙烯酸辛酯等。以辛醇为原料生产的邻苯二甲酸二辛酯是PVC的主要增塑剂,用于PVC的消耗量约占其总消费量的95%。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-Aethyl-hexyl-hydroperoxid-(3) 90951-85-2 C8H18O2 146.23
反应信息
-
作为反应物:参考文献:名称:Masson, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1901, vol. 132, p. 484摘要:DOI:
-
作为产物:参考文献:名称:锆茂-烯烃配合物与醛或酮的高度区域选择性反应摘要:的锆-烯烃络合物的Cp反应2的Zr(CH 2 CHR)(PR' 3)(RH,ME等,先生“ 3或Ar)与醛或酮的关系进行了研究。锆茂-乙烯,-丙烯或1-丁烯络合物在烯烃的末端碳上与醛或酮反应,在水解后具有高区域选择性,得到相应的醇。氧化锆环戊烷与醛的反应也获得了相似类型的反应产物。该反应通过锆碳环戊烷的β-β'碳-碳键裂解进行。锆茂-乙烯基硅烷络合物与酮的反应得到具有优异区域选择性的3-三甲基甲硅烷基-1-氧杂-2-氧化锆环戊烷。碳-碳键的形成仅发生在乙烯基硅烷的末端碳上。水解后得到它们相应的γ-甲硅烷基醇。产物表明,乙烯基硅烷与β-碳上的羰基化合物反应生成甲硅烷基。它与乙烯基硅烷的常规反应形成鲜明对比,后者的α-碳通常会侵蚀亲电体。苯乙烯及其衍生物与戊烷-3-酮在锆上的反应产生了两种区域异构体的混合物。在1-氧杂-2-氧化锆环戊烷中,烯烃的取代基倾向于位于Zr的α位。该取向显示了由锆DOI:10.1016/0022-328x(94)80112-6
文献信息
-
SLI381 (Adderall XR), a Two-Component, Extended-Release Formulation of Mixed Amphetamine Salts: Bioavailability of Three Test Formulations and Comparison of Fasted, Fed, and Sprinkled Administration作者:Simon J. Tulloch、Yuxin Zhang、Angus McLean、Kathleen N. WolfDOI:10.1592/phco.22.16.1405.33687日期:2002.11Study Objectives. To assess the bioavailability of three test formulations of a single dose of extended-release Adderall 20-mg capsules compared with two doses of immediate-release Adderall 10-mg tablets, and to assess the bioequivalence of a single 30-mg dose of the chosen extended-release Adderall formulation (designated as SLI381) administered in applesauce (sprinkled) and the same dose administered as an intact capsule with or without food. Design. Randomized, open-label, crossover study. Setting. Clinical research unit. Patients. Forty-one healthy adults. Interventions. Study A had four treatment sequences: three test formulations (A, B, and C) of a single dose of extended-release Adderall 20 mg, and two 10-mg doses of Adderall given 4 hours apart. Study B had three treatment sequences: a single dose of SLI381 30 mg as an intact capsule after overnight fast, an intact capsule after a high-fat breakfast, and the contents of a capsule sprinkled in 1 tablespoon of applesauce. Measurements and Main Results. The 20-mg test formulation A had comparable pharmacokinetic profiles and bioequivalence in rate and extent of drug absorption to Adderall 10 mg twice/day for both d- and l-amphetamine. Formulations B and C had statistically significant differences from the reference drug in some pharmacokinetic parameters. A 30-mg dose of SLI381 showed no significant differences in rate and extent of absorption of d- and l-amphetamine for fasted or sprinkled conditions compared with the high-fat meal condition. Conclusion. SLI381 20 mg/day is bioequivalent to Adderall 10 mg twice/day. SLI381 30 mg administered in applesauce is bioequivalent in terms of both rate and extent of absorption to the same dose administered as an intact capsule in both fasted and fed states.研究目的:评估单剂量缓释Adderall 20毫克胶囊的三种试验制剂与两剂量即释Adderall 10毫克片剂的生物利用度,并评估所选择的缓释Adderall 30毫克单剂量制剂(指定为SLI381)以苹果泥(撒粉)形式给药与相同剂量完整胶囊给药(有无食物)的生物等效性。设计:随机、开放标签、交叉研究。环境:临床研究中心。对象:41名健康成人。干预:研究A有四个治疗序列:三种试验制剂(A、B、C)的单剂量缓释Adderall 20毫克,以及间隔4小时的两剂10毫克Adderall。研究B有三个治疗序列:过夜禁食后单剂SLI381 30毫克完整胶囊,高脂早餐后完整胶囊,以及1汤匙苹果泥中撒粉的胶囊内容物。测量与主要结果:20毫克试验制剂A在d-和l-安非他命的药物动力学曲线与生物等效性方面与Adderall 10毫克每日两次相当。制剂B和C在某些药物动力学参数上与参照药物存在统计学显著差异。SLI381 30毫克在d-和l-安非他命的吸收速率和程度方面,禁食或撒粉条件下与高脂餐条件下无显著差异。结论:SLI381 20毫克每日一次与Adderall 10毫克每日两次生物等效。SLI381 30毫克以苹果泥形式给药在吸收速率和程度方面与禁食和进食状态下完整胶囊给药等效。
-
OPTICAL RECORDING MEDIUM AND COMPOUND USED FOR THE SAME申请人:SHIOZAKI Hiroyoshi公开号:US20090306376A1公开(公告)日:2009-12-10A compound comprising a ring structure including a ring composed of four carbon atoms and two nitrogen atoms and a substituted or unsubstituted amino group bonded to the ring structure.一种化合物,包括一个环结构,其中包括由四个碳原子和两个氮原子组成的环,以及与环结构相结合的取代或未取代的氨基团。
-
[EN] PROCESS<br/>[FR] PROCÉDÉ申请人:PHOSPHAGENICS LTD公开号:WO2018112512A1公开(公告)日:2018-06-28An efficient and commercial phosphorylation process of a complex alcohol, such as secondary and tertiary alcohols, with P4O10 at high temperatures, and a product obtained by the process.
-
Substituted Azaspiro(4.5)Decane Derivatives申请人:Gruenenthal GmbH公开号:US20160016903A1公开(公告)日:2016-01-21The invention relates to substituted spirocyclic cyclohexane derivatives which have an affinity for the μ opioid receptor and the ORL1 receptor, processes for the preparation thereof, medicaments containing these compounds and the use of these compounds for the preparation of medicaments.
-
FULLERENE DERIVATIVE AND n-TYPE SEMICONDUCTOR MATERIAL申请人:DAIKIN INDUSTRIES, LTD.公开号:US20180282274A1公开(公告)日:2018-10-04An object of the present invention is to provide a novel fullerene derivative usable in n-type semiconductor materials for organic thin-film solar cells and the like. The object is achieved by a fullerene derivative represented by formula (1) wherein R 1 represents aryl optionally substituted with at least one substituent, R 2 represents an organic group, R 3 represents an organic group, with the proviso that at least one of R 2 and R 3 is alkyl optionally substituted with at least one substituent or alkyl ether optionally substituted with at least one substituent, R 4 represents hydrogen or an organic group, and a ring A represents a fullerene ring.
表征谱图
-
氢谱1HNMR
-
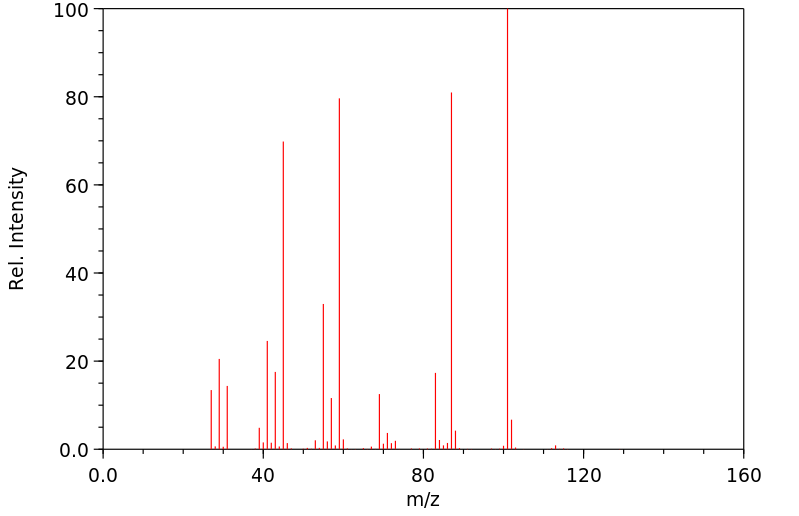
质谱MS
-
碳谱13CNMR
-
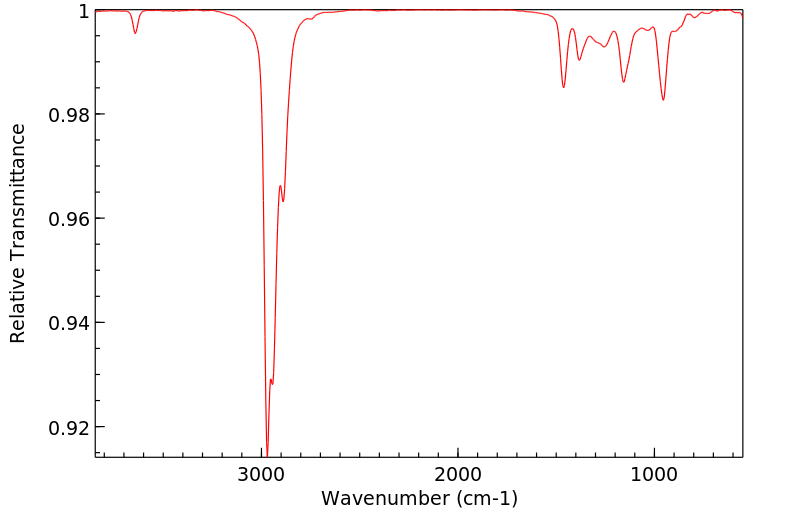
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息