环15烷 | 295-48-7
中文名称
环15烷
中文别名
环十五烷
英文名称
cyclopentadecane
英文别名
Cyclopentadecan
CAS
295-48-7
化学式
C15H30
mdl
MFCD00039424
分子量
210.403
InChiKey
SRONXYPFSAKOGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64 °C
-
沸点:284.95°C (rough estimate)
-
密度:0.805
-
溶解度:溶于丙酮
-
保留指数:1550;1522
计算性质
-
辛醇/水分配系数(LogP):8.3
-
重原子数:15
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险品标志:Xn,Xi
-
安全说明:S26,S27,S36/37,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
危险品运输编号:3276
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
环十五烷 修改号码:5
模块 1. 化学品
产品名称: CyclopeNTadecane
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 环十五烷
百分比: >98.0%(GC)
CAS编码: 295-48-7
分子式: C15H30
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
环十五烷 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色
气味: 无资料
pH: 无数据资料
熔点:
64°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 丙酮
环十五烷 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
环十五烷 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: CyclopeNTadecane
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 环十五烷
百分比: >98.0%(GC)
CAS编码: 295-48-7
分子式: C15H30
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
环十五烷 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色
气味: 无资料
pH: 无数据资料
熔点:
64°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 丙酮
环十五烷 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
环十五烷 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 环辛烷 Cyclooctan 292-64-8 C8H16 112.215
反应信息
-
作为反应物:描述:环15烷 在 十二羰基三钌 、 2-碘-1,3,5-三甲基苯 、 N,N'-1,2-tetrakis(4-fluorophenyl)ethane-1,2-diimine 、 caesium carbonate 作用下, 以 氯苯 为溶剂, 反应 24.0h, 以91%的产率得到cyclopentadecene参考文献:名称:通过分子间氢原子转移机制的钌催化脱氢摘要:烷烃直接脱氢是获得有价值的烯烃产品的最有效方法之一。尽管已经设计了几种催化剂来促进这种转变,但不幸的是它们在精细化学合成中的应用有限。在这里,我们报告了一种使用钌催化剂催化烷烃分子间脱氢的概念新颖的策略。氧化还原活性配体和空间位阻芳基自由基中间体的组合释放了这种新策略。重要的是,已经进行了机理研究,为进一步开发这种新型催化脱氢系统提供了概念框架。DOI:10.1002/anie.202015837
-
作为产物:描述:参考文献:名称:Muehlstaedt,M.; Graefe,J., Chemische Berichte, 1967, vol. 100, p. 223 - 227摘要:DOI:
文献信息
-
Direct C–C Bond Formation from Alkanes Using Ni-Photoredox Catalysis作者:Laura K. G. Ackerman、Jesus I. Martinez Alvarado、Abigail G. DoyleDOI:10.1021/jacs.8b09191日期:2018.10.31C(sp3)-H bonds and chloroformates has been accomplished via nickel and photoredox catalysis. A diverse range of feedstock chemicals, such as (a)cyclic alkanes and toluenes, along with late-stage intermediates, undergo intermolecular C-C bond formation to afford esters under mild conditions using only 3 equiv of the C-H partner. Site selectivity is predictable according to bond strength and polarity
-
Highly Regioselective Amination of Unactivated Alkanes by Hypervalent Sulfonylimino-λ <sup>3</sup> -Bromane作者:Masahito Ochiai、Kazunori Miyamoto、Takao Kaneaki、Satoko Hayashi、Waro NakanishiDOI:10.1126/science.1201686日期:2011.4.22to afford triflyl-substituted amines in moderate to high yields. Marked selectivity for tertiary over secondary C–H bonds was observed; primary (methyl) C–H bonds were inert. Addition of hexafluoroisopropanol to inhibit decomposition of 1 dramatically improved the C–H amination efficiencies. Second-order kinetics, activation parameters (negative activation entropy), deuterium isotope effects, and theoretical
-
Multiple deuteration of alkanes synergistically-catalyzed by platinum and rhodium on carbon as a mixed catalytic system作者:Tsuyoshi Yamada、Yoshinari Sawama、Kyoshiro Shibata、Kosuke Morita、Yasunari Monguchi、Hironao SajikiDOI:10.1039/c4ra16386a日期:——accomplished an efficient and mild multiple deuteration method for alkanes catalyzed by the combined use of heterogeneous platinum on carbon (Pt/C) and rhodium on carbon (Rh/C) catalysts in i-PrOD-d8 and D2O as a mixed solvent. The present multi-deuteration could be initiated by the transition metal-catalyzed dedeuteration of i-PrOD-d8 to produce D2 and the subsequent C–H bond activation of alkanes catalyzed
-
CIRCULAR ECONOMY METHODS OF PREPARING UNSATURATED COMPOUNDS申请人:INTERNATIONAL FLAVORS & FRAGRANCES INC.公开号:US20170362155A1公开(公告)日:2017-12-21Methods of preparing unsaturated compounds or analogs through dehydrogenation of corresponding saturated compounds and/or hydrogenation of aromatic compounds are disclosed.通过脱氢饱和化合物和/或芳香化合物氢化的方法制备不饱和化合物或类似物已被披露。
-
The chemistry of thiophene-based bis-(p-quinodimethanes): an approach to macrocycles作者:Walter S. Trahanovsky、Douglas A. KlumppDOI:10.1016/j.tetlet.2016.04.062日期:2016.6Bis-2,5-dimethylene-2,5-dihydrothiophenes have been generated in the gas-phase by flash vacuum pyrolysis (FVP) of diester precursors. These thiophene-based bis-(p-quinodimethanes) are shown to undergo reactions leading to macrocycles. The conversions are consistent with a mechanism involving cyclic diradical intermediates followed by disproportionation of the radical centers.通过二酯前体的快速真空热解(FVP)在气相中生成了Bis-2,5-二亚甲基-2,5-二氢噻吩。这些基于噻吩的双-(对-喹二甲烷)显示出发生导致大环的反应。所述转化与涉及环状双自由基中间体然后自由基中心歧化的机理是一致的。
表征谱图
-
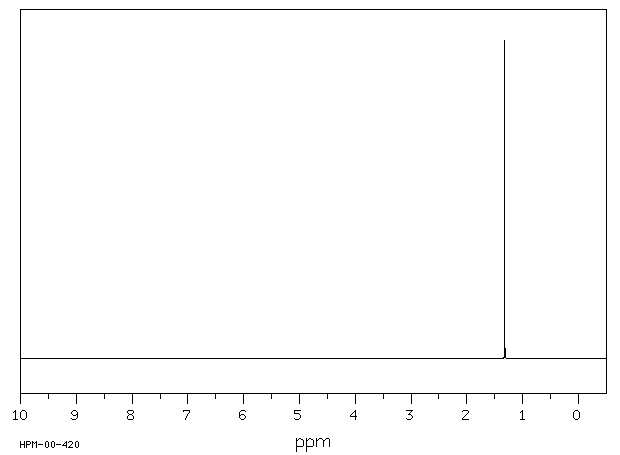
氢谱1HNMR
-
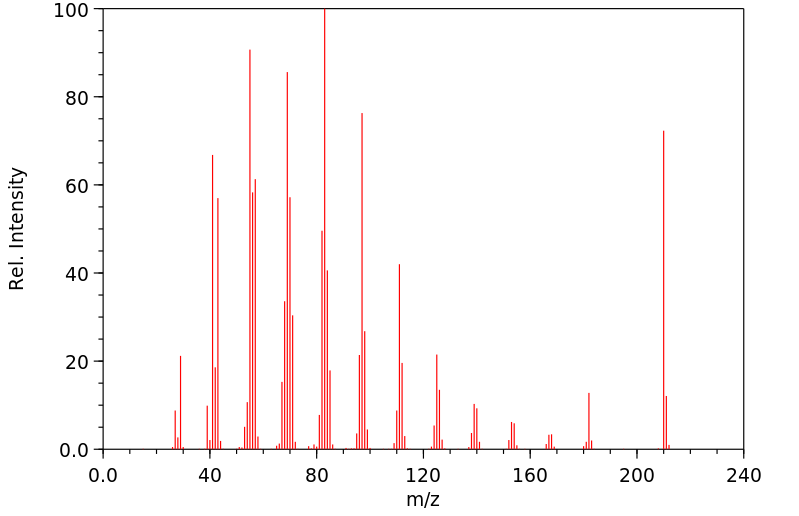
质谱MS
-
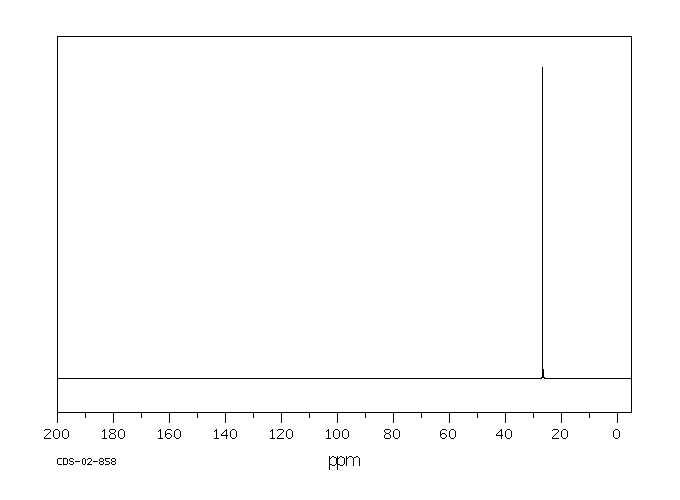
碳谱13CNMR
-
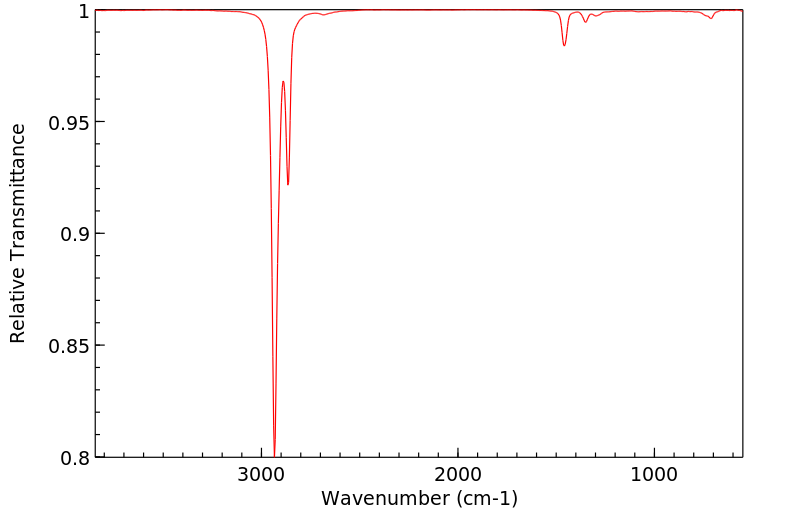
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷