癸烷 | 124-18-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-30 °C
-
沸点:174 °C(lit.)
-
密度:0.735
-
蒸气密度:4.9 (vs air)
-
闪点:115 °F
-
溶解度:0.00005克/升
-
介电常数:1.8(130℃)
-
LogP:5.010
-
物理描述:N-decane appears as a colorless liquid. Flash point 115°F. Less dense than water and insoluble in water. Vapors heavier than air. In high concentrations its vapors may be narcotic. Used as a solvent and to make other chemicals.
-
颜色/状态:Colorless liquid
-
蒸汽密度:4.9 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1.43 mm Hg at 25 °C
-
大气OH速率常数:1.16e-11 cm3/molecule*sec
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强酸、强碱、卤素。
-
聚合危害:不聚合。
-
-
自燃温度:410 °F (210 °C)
-
粘度:2.188 mPa s at -25 °C; 1.277 mPa s at 0 °C; 0.838 mPa s at 25 °C; 0.598 mPa s at 50 °C; 0.453 mPa s at 75 °C; 0.359 mPa s at 100 °C
-
燃烧热:-6778.29 kJ/mol at 25 °C
-
汽化热:39.58 kJ/mol at 174.15 °C; 51.42 kJ/mol at 25 °C
-
表面张力:24.75 mN/m at 10 °C; 23.37 mN/m at 25 °C; 21.07 mN/m at 50 °C; 18.77 mN/m at 75 °C; 16.47 mN/m at 100 °C
-
气味阈值:11 mg/cu m
-
折光率:Index of refraction: 1.4102 at 20 °C
-
相对蒸发率:First-order evaporation constants of n-decane in 3-mm layer No 2 fuel oil, darkened room, wind speed: 21 km/hr: at 5 °C, 1.19X10-3/min; at 10 °C, 1.87X10-3/min; at 20 °C, 3.44X10-3/min; at 30 °C, 6.98X10-3/min
-
保留指数:1000
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:10
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xn,F,N
-
安全说明:S16,S23,S29,S33,S36/37,S61,S62,S9
-
危险类别码:R67,R38,R66,R10,R11,R51/53,R62,R65,R48/20
-
WGK Germany:3
-
海关编码:29011090
-
危险品运输编号:UN 2247 3/PG 3
-
危险类别:3
-
RTECS号:HD6550000
-
包装等级:III
-
危险标志:GHS02,GHS08
-
危险性描述:H226,H304
-
危险性防范说明:P301 + P310,P331
-
储存条件:储存注意事项: - 储存在阴凉、通风的库房中。 - 远离火源和热源,库温不宜超过37℃。 - 保持容器密封。 - 应与氧化剂分开存放,切忌混储。 - 使用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 33506 |
| CAS: | 124-18-5 |
| 中文名称: | 正癸烷 |
| 英文名称: | decane;n-decyl hydride |
| 别 名: | 十碳烷 |
| 分子式: | C 10 H 22 ;CH 3 (CH 2 ) 8 CH 3 |
| 分子量: | 142.29 |
| 熔 点: | -29.7℃ |
| 密 度: | 相对密度(水=1)0.73; |
| 蒸汽压: | 46℃ |
| 溶解性: | 不溶于水,可混溶于乙醇、乙醚 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体 |
| 危险标记: | 7(易燃液体) |
| 用 途: | 用作溶剂,及用于有机合成,也用于燃料研究 |
2.对环境的影响: 一、健康危害 侵入途径:吸入、食入、经皮吸收。 健康危害:吸入、口或经皮肤吸收对身体有害。其蒸气或雾对眼睛、皮肤、粘膜和呼吸道有刺激作用。吸入后可引起化学性肺炎、肺水肿。 二、毒理学资料及环境行为 急性毒性:LD506.4~12.8g/kg(小鼠经口);12.8~25.6g/kg(大鼠经口);LC5072300mg/m3,2小时(小鼠吸入) 致癌性:小鼠经皮最低中毒剂量(TDL0):25g/kg(52周,间歇),致肿瘤阳性。 危险特性:易燃,其蒸气与空气可形成爆炸性混合物。遇高热、明火能引起燃烧爆炸。与氧化剂能发生强烈反应。在火场中,受热的容器有爆炸危险。 燃烧(分解)产物:一氧化碳、二氧化碳。 3.现场应急监测方法: 4.实验室监测方法: 气相色谱法《空气中有害物质的测定方法》(第二版),杭士平主编 5.环境标准: 前苏联 车间空气中有害物质的最高容许浓度 10mg/m3 嗅觉阈浓度 6.3ppb 6.应急处理处置方法: 一、泄漏应急处理 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 二、防护措施 呼吸系统防护:空气中浓度较高时,应该佩戴自吸过滤式防毒面具(半面罩)。 眼睛防护:戴安全防护眼镜。 身体防护:穿防静电工作服。 手防护:戴防苯耐油手套。 其它:工作现场严禁吸烟。避免长期反复接触。 三、急救措施 皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。 眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 食入:饮足量水,催吐。就医。 灭火方法:尽可能将容器从火场移至空旷处。灭火剂:泡沫、二氧化碳、干粉、砂土。用水灭火无效,但须用水冷却火场容器。用雾状水保护消防人员,用砂土堵逸出液体。
制备方法与用途
癸烷是一种无色液体,熔点为-30℃,沸点为174℃,相对密度为0.7301(20/4℃),折光率为1.4114。它在空气中较为稳定,在高温下会燃烧。癸烷能溶解于烃类溶剂、石蜡油和苯中,但不溶于水,并存在于石蜡基石油中。癸烷与其他高级烷烃的混合物可作为凡士林、润滑剂及其他化工产品的原料。
化学性质癸烷是一种无色液体,熔点为-30℃,沸点为174℃,相对密度为0.7301(20/4℃),折光率为1.4114,闪点为46℃。它能与醇和醚混溶,不溶于水。
用途癸烷主要用于有机合成和燃料研究,并可用作中沸点溶剂,适用于仪器洗涤、干洗及印刷油墨的无臭溶剂。
生产方法 类别- 易燃液体
- 毒性分级:低毒
- 急性毒性:吸入 - 小鼠 LC50: 72300 毫克/立方米/2小时
癸烷与空气混合可爆炸。
可燃性危险特性遇到明火、高温或氧化剂时较易燃,燃烧过程中会产生刺激烟雾。
储运特性存放于通风低温干燥的库房中,并应与其他氧化剂分开存放。
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 环癸烷 cyclodecane 293-96-9 C10H20 140.269 十二烷 dodecane 112-40-3 C12H26 170.338 正十六烷 Hexadecane 544-76-3 C16H34 226.446 正辛烷 octane 111-65-9 C8H18 114.231 正十八烷 octadecane 593-45-3 C18H38 254.5 正庚烷 n-heptane 142-82-5 C7H16 100.204 正己烷 hexane 110-54-3 C6H14 86.1772 正戊烷 pentane 109-66-0 C5H12 72.1503 1,10-二碘癸烷 1,10-diiododecane 16355-92-3 C10H20I2 394.078 1-碘癸烷 Iododecane 2050-77-3 C10H21I 268.181 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 分析纯正二十烷 icosane 112-95-8 C20H42 282.553 正辛烷 octane 111-65-9 C8H18 114.231 正壬烷 nonane 111-84-2 C9H20 128.258 十一烷 Undecan 1120-21-4 C11H24 156.312 十二烷 dodecane 112-40-3 C12H26 170.338 正三十三烷 tritriacontane 630-05-7 C33H68 464.903 正三十四烷 n-tetratriacontane 14167-59-0 C34H70 478.93 正十五烷 pentadecane 629-62-9 C15H32 212.419 二十二烷 n-docosane 629-97-0 C22H46 310.607 十三烷 Tridecane 629-50-5 C13H28 184.365 十四烷 tetradecane 629-59-4 C14H30 198.392 正十六烷 Hexadecane 544-76-3 C16H34 226.446 正十七烷 hepatdecane 629-78-7 C17H36 240.473 正十八烷 octadecane 593-45-3 C18H38 254.5 正十九烷 n-nonadecane 629-92-5 C19H40 268.527 正二十一烷 heneicosane 629-94-7 C21H44 296.58 正二十六烷 n-hexacosane 630-01-3 C26H54 366.715 正二十七烷 heptacosane 593-49-7 C27H56 380.742 正三十一烷 hentriacontane 630-04-6 C31H64 436.849 正二十九烷 nonacosane 630-03-5 C29H60 408.795 正二十三烷 tricosane 638-67-5 C23H48 324.634 正二十四烷 tetracosane 646-31-1 C24H50 338.661 二十五烷 pentacosane 629-99-2 C25H52 352.688 二十八烷 octacosane 630-02-4 C28H58 394.769 三十烷 n-triacontane 638-68-6 C30H62 422.822 正三十二烷 dotricontane 544-85-4 C32H66 450.876 正庚烷 n-heptane 142-82-5 C7H16 100.204 正己烷 hexane 110-54-3 C6H14 86.1772 矿油精 isodecane 871-83-0 C10H22 142.285 正戊烷 pentane 109-66-0 C5H12 72.1503 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:Regioselective ω-hydroxylation of medium-chain n-alkanes and primary alcohols by CYP153 enzymes from Mycobacterium marinum and Polaromonas sp. strain JS666摘要:饱和烃的氧官能化是基础和应用化学中的一个重要目标。生物催化剂如细胞色素P450酶可以以非常选择性的方式将氧引入多种分子中,这可以用于精细化学品和大宗化学品的合成。来自CYP153A亚家族的细胞色素P450酶被描述为具有高末端区域选择性的烷烃羟化酶。在这里,我们报告了由来自海洋分枝杆菌(CYP153A16)和极地单胞菌(CYP153A P. sp.)的CYP153A酶催化的C5–C12烷烃和醇氧化反应的产物产率。对于所有反应,副产物的形成进行了详细描述。经过在大肠杆菌中克隆和表达后,纯化的单氧化酶的活性与变色龙还原蛋白(CamA)和变色龙还原酶(CamB)重新结合。尽管这两种酶系统都产生初级醇和α,ω-烷二醇,但它们对烷烃的氧化模式却有所不同。对于CYP153A P. sp.,观察到主要的ω-羟化活性,而CYP153A16则具备催化ω-羟化和α,ω-二羟化反应的能力。DOI:10.1039/c1ob05565h
-
作为产物:参考文献:名称:使用9,9-二正丁基-9-硼环[3.3.1]壬酸锂将叔烷基,苄基和烯丙基卤选择性还原为烃摘要:标题9-borabicyclo [3.3.1]壬烷(9-BBN)配合物(1)可以选择性地除去叔烷基,苄基和烯丙基卤,从而以优异的收率得到相应的烃,而不会伴随着对仲,伯和芳基的攻击衍生品。的还原顺式-和反式- 4 -吨-丁基- 1 -甲基环己基氯化物(2)与1给出4 -吨-丁基- 1 - methylcyclohexanes(3),在环己烷的配置的局部反转,而在苯给出热力学稳定的反式- 3为主。1,1-二甲基-5-己烯基氯化物的反应(4)和1,7,7- -三甲基二环[2.2.1]庚- 2 -基磺酰氯(8)配有1继续进行到碳离子中间特性的重排。要求1所述的还原-乙基- 1 -甲基戊基氯与1如下二阶速率方程。DOI:10.1016/s0040-4020(01)97982-7
-
作为试剂:描述:2,4,4-三甲基-2-戊烯 在 MnII-bis-(1,10-phenanthroline) templated SBA-15 过氧乙酸 、 癸烷 作用下, 以 乙腈 为溶剂, 反应 0.17h, 生成 2,3-环氧-2,4,4-三甲基戊烷参考文献:名称:通过金属模板/金属交换方法对离散的 Mn(II) 双苯配合物进行共价异质化:一种具有增强反应性的环氧化催化剂摘要:由于位点隔离可能带来的好处,例如增加催化剂稳定性、催化剂回收和产品分离,将分散的环氧化催化剂固定在固体载体上引起了相当大的关注。本文报道了一种合成金属模板/金属交换方法,可将共价连接的双-1,10-菲咯啉配位环境印记到高表面积介孔 SBA-15 二氧化硅上,并在重新加载锰后具有环氧化反应性。这种印迹材料与通过配体随机接枝合成的材料的比较表明,模板方法在各种配体负载下产生了更具重现性的、类似溶液的双 1,10-菲咯啉配位。烯烃与过乙酸的环氧化表明,印迹锰催化剂对环氧化物的产物选择性有所提高,底物范围更大,氧化剂的使用更有效,并且比其均相或接枝类似物具有更高的反应性,而不受配体负载的影响。然而,随机接枝的锰催化剂显示出随着配体负载而变化的反应性,而均相类似物降解三取代的烯烃并从顺式烯烃产生反式环氧化物产物。模板化催化剂的有效回收行为也是可能的。显示反应性随配体负载而变化,而均相类似物降解三取代DOI:10.1021/ja0742030
文献信息
-
Application of Pd Nanoparticles Supported on Mesoporous Hollow Silica Nanospheres for the Efficient and Selective Semihydrogenation of Alkynes作者:Oscar Verho、Haoquan Zheng、Karl P. J. Gustafson、Anuja Nagendiran、Xiaodong Zou、Jan-E. BäckvallDOI:10.1002/cctc.201501112日期:2016.2Herein, the preparation of a heterogeneous catalyst consisting of 1–2 nm sized Pd nanoparticles supported on amino‐functionalized mesoporous hollow silica nanospheres and its use for the semihydrogenation of mono‐ and disubstituted alkynes is reported. By utilizing this Pd nanocatalyst together with the green poisoning agent DMSO, high yields of the desired alkenes could be achieved, while suppressing
-
氢化反应方法申请人:郑州大学公开号:CN111099986B公开(公告)日:2023-02-03
-
Ambient Hydrogenation and Deuteration of Alkenes Using a Nanostructured Ni‐Core–Shell Catalyst作者:Jie Gao、Rui Ma、Lu Feng、Yuefeng Liu、Ralf Jackstell、Rajenahally V. Jagadeesh、Matthias BellerDOI:10.1002/anie.202105492日期:2021.8.16selective hydrogenation and deuteration of a variety of alkenes is presented. Key to success for these reactions is the use of a specific nickel-graphitic shell-based core–shell-structured catalyst, which is conveniently prepared by impregnation and subsequent calcination of nickel nitrate on carbon at 450 °C under argon. Applying this nanostructured catalyst, both terminal and internal alkenes, which
-
Iron-Catalyzed Cross-Coupling of Unactivated Secondary Alkyl Thio Ethers and Sulfones with Aryl Grignard Reagents作者:Scott E. Denmark、Alexander J. CresswellDOI:10.1021/jo402246h日期:2013.12.20ed cross-coupling are described. Initial studies focused on discerning the structural and electronic features of the organosulfur substrate that enable the challenging oxidative addition to the C(sp3)–S bond. Through extensive optimization efforts, an Fe(acac)3-catalyzed cross-coupling of unactivated alkyl aryl thio ethers with aryl Grignard reagents was realized in which a nitrogen “directing group”描述了未活化的脂肪族硫化合物作为过渡金属催化交叉偶联中的亲电试剂的首次系统研究。最初的研究侧重于识别有机硫底物的结构和电子特征,这些特征能够对 C(sp 3 )-S 键进行具有挑战性的氧化加成。通过广泛的优化工作,实现了未活化的烷基芳基硫醚与芳基格氏试剂的 Fe(acac) 3催化交叉偶联,其中硫醚 S-芳基部分上的氮“导向基团”起到了关键作用促进氧化加成步骤。此外,发现烷基苯砜是 Fe(acac) 3 中有效的亲电试剂。-催化与芳基格氏试剂的交叉偶联。对于后一类亲电试剂,对各种反应参数的彻底评估表明,过量的 TMEDA(8.0 当量)反应效率显着提高。优化的反应方案用于评估该方法在有机镁亲核试剂和砜亲电试剂方面的范围。
-
Iridium-Catalyzed Direct Dehydroxylation of Alcohols作者:Jian-Lin Huang、Xi-Jie Dai、Chao-Jun LiDOI:10.1002/ejoc.201301293日期:2013.10Iridium-catalyzed direct dehydroxylation of alcohols with hydrazine was developed through a combination of the oxidation of alcohols and the Wolff–Kishner reduction. This protocol is simple to perform and highly efficient for a series of primary, benzylic and allylic alcohols.
表征谱图
-
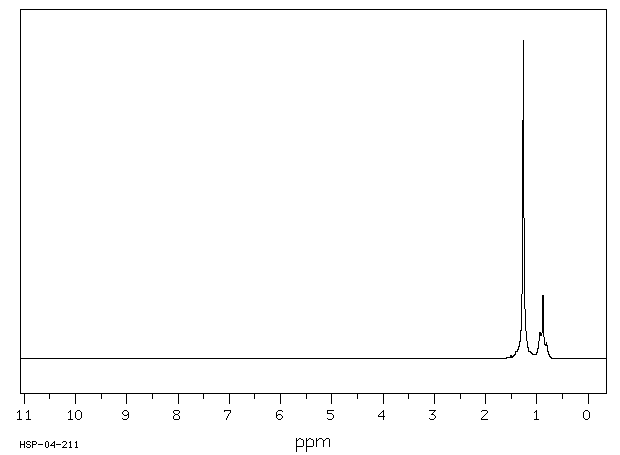
氢谱1HNMR
-
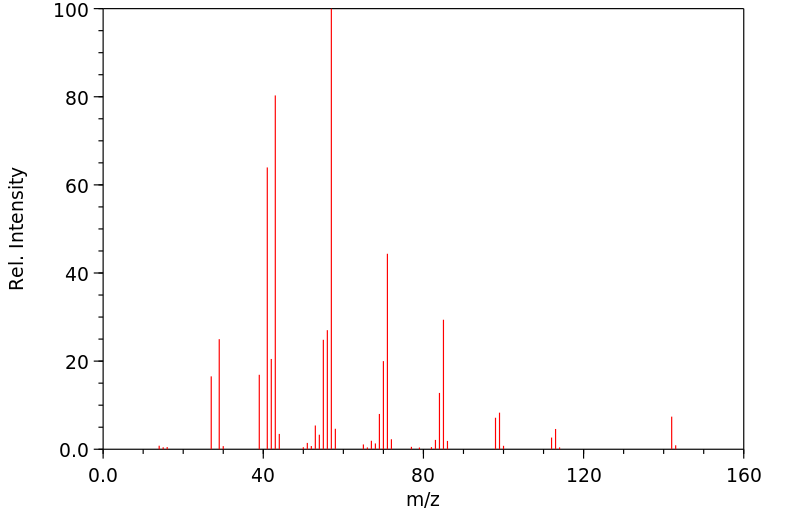
质谱MS
-
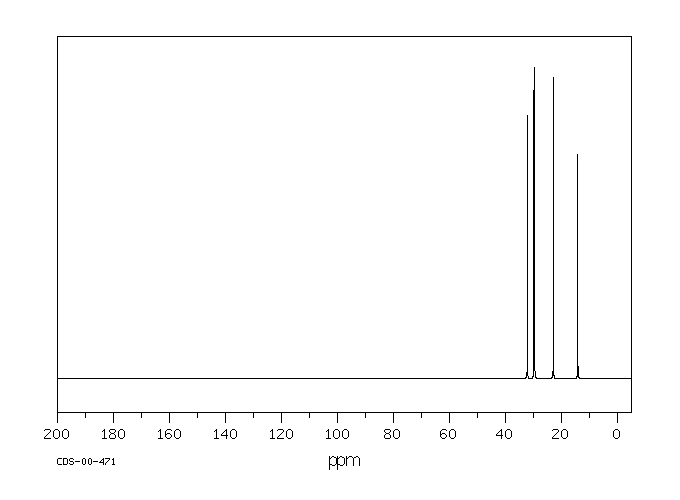
碳谱13CNMR
-
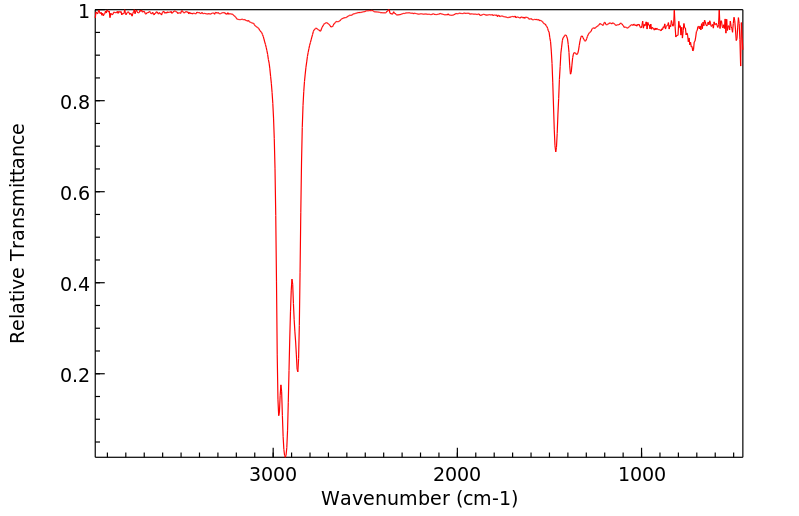
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息