四氢二环戊二烯 | 6004-38-2
中文名称
四氢二环戊二烯
中文别名
四氢二聚环戊二烯
英文名称
endo-octahydro-4,7-methano-1H-indene
英文别名
endo-tricyclo[5.2.1.02,6]decane;exo-tetrahydrodicyclopentadiene;Tetrahydrodicyclopentadiene;tricyclo[5.2.1.02,6]decane
CAS
6004-38-2
化学式
C10H16
mdl
——
分子量
136.237
InChiKey
LPSXSORODABQKT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75°C
-
沸点:185.55°C (rough estimate)
-
密度:0.8455 (estimate)
-
溶解度:可溶于氯仿(少量)、DMSO(少量)
-
LogP:4.220 (est)
-
物理描述:Liquid
-
保留指数:1077.6;1078
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:10
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
SDS
| Name: | Tricyclo(5.2.1.0/2 6)decane 98% Material Safety Data Sheet |
| Synonym: | Tetrahydrodicyclopentadien |
| CAS: | 6004-38-2 |
Synonym:Tetrahydrodicyclopentadien
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6004-38-2 | Tricyclo[5.2.1.0/2,6]decane, 98% | 98 | 227-851-2 |
Risk Phrases: 11
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Highly flammable.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Water Reactive. Material will react with water and may release a flammable and/or toxic gas.
Extinguishing Media:
Do NOT use water directly on fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Keep away from water. Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 6004-38-2: Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Wear a NIOSH/MSHA or European Standard EN 149 approved full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Color: colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: 172 20.00 deg C
Boiling Point: 193 deg C @ 769.00mm Hg
Freezing/Melting Point: 77.00 - 79.00 deg C
Autoignition Temperature: 275 deg C ( 527.00 deg F)
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H16
Molecular Weight: 136.24
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6004-38-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tricyclo[5.2.1.0/2,6]decane, 98% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE SOLID, ORGANIC, N.O.S.*
Hazard Class: 4.1
UN Number: 1325
Packing Group: II
IMO
Shipping Name: FLAMMABLE SOLID, ORGANIC, N.O.S.
Hazard Class: 4.1
UN Number: 1325
Packing Group: II
RID/ADR
Shipping Name: FLAMMABLE SOLID, ORGANIC, N.O.S.
Hazard Class: 4.1
UN Number: 1325
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: F
Risk Phrases:
R 11 Highly flammable.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
WGK (Water Danger/Protection)
CAS# 6004-38-2: No information available.
Canada
CAS# 6004-38-2 is listed on Canada's DSL List.
CAS# 6004-38-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6004-38-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— Diammonal 91967-78-1 C11H16O 164.247 八氢-4,7-甲桥-1H-茚-2,5-二甲醇 4,8-bis(hydroxymethyl)tricyclo[5.2.1.0.2.6]decane 28132-01-6 C12H20O2 196.29 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— protoadamantane 19026-94-9 C10H16 136.237 金刚烷 adamantane 281-23-2 C10H16 136.237
反应信息
-
作为反应物:参考文献:名称:Y-沸石上四氢环戊二烯向金刚烷的催化重排摘要:双功能稀土交换Y型沸石,在固定床流动系统中,在氢气和氯化氢存在下,对四氢双环戊二烯合成金刚烷具有高催化活性。因沉积烃堵塞孔隙而失活的催化剂几乎可以通过加氢裂化完全再生。DOI:10.1246/cl.1986.315
-
作为产物:描述:dicyclopentadiene 在 sodium tetrahydroborate 、 氢气 、 nickel dichloride 作用下, 以 异丙醇 为溶剂, 40.0 ℃ 、101.33 kPa 条件下, 反应 6.0h, 以95%的产率得到四氢二环戊二烯参考文献:名称:Hydrogenation of alkenes over nickel nanoparticles under atmospheric pressure of hydrogen摘要:Nickel nanoparticles have been shown to be an accessible catalyst which allows hydrogenation of unsaturated compounds to be accomplished under atmospheric pressure of hydrogen at relatively low temperatures. Linear and cyclic alkenes, styrene and norbornene derivatives, as well as pinenes and camphene have been smoothly hydrogenated under these conditions. In some cases, selective hydrogenation of unsaturated carbon-carbon bond is possible with the other functional group remaining intact.DOI:10.1134/s1070428016030040
文献信息
-
The NiCl2-Li-Arene (cat.) Combination as Reducing System, Part 9: Catalytic Hydrogenation of Organic Compounds using the NiCl2-Li-(Naphthalene or Polymer-Supported Naphthalene) (cat.) Combination作者:Francisco Alonso、Pablo Candela、Cecilia Gómez、Miguel YusDOI:10.1002/adsc.200390022日期:2003.1polymer-supported naphthalene, and anhydrous nickel(II) chloride, in THF at room temperature, generates a finely divided and very reactive nickel(0) which has been efficiently applied to the catalytic hydrogenation of different organic compounds such as alkenes, alkynes, carbonyl compounds, imines, organic halides, aromatic compounds, hydrazines, azoxy compounds, and N-oxides.
-
Catalyst-Free Hydrogenation of Alkenes and Alkynes with Hydrazine in the Presence of Oxygen作者:Metin Balci、Nurettin MengesDOI:10.1055/s-0033-1340554日期:——A series of alkenes and alkynes was subjected to reduction with hydrazine hydrate in ethanol in the presence of oxygen. An efficient method was developed for the reduction of C–C double bonds and C–C triple bonds with diimide, generated in situ from hydrazine hydrate by oxidation with oxygen. The reduction process proceeded for 24–48 hours with high chemoselectivity and excellent yields. This reduction
-
Dendrimer‐Encapsulated Pd Nanoparticles, Immobilized in Silica Pores, as Catalysts for Selective Hydrogenation of Unsaturated Compounds作者:Edward A. Karakanov、Anna V. Zolotukhina、Andrey O. Ivanov、Anton L. MaximovDOI:10.1002/open.201800280日期:2019.3on the presence of Si(OEt)4, while dendrimer molecules acting as both anchored ligands and template, demonstrated the maximum activity in the hydrogenation of terminal linear alkynes and conjugated dienes, reaching TOF values up to 400000 h−1. Herein the total selectivity on alkene in the case of terminal alkynes and conjugated dienes reached 95–99 % even at hydrogen pressure of 30 atm. The catalysts通过原位共水解获得的基于固定在二氧化硅孔和网络中的聚(丙烯亚胺)树枝状聚合物的多相含钯纳米催化剂已被合成并在各种不饱和化合物的氢化中进行了检验。发现催化剂活性和选择性强烈依赖于载体结构以及底物电子和几何特征。因此,在聚合物模板和四乙氧基硅烷存在下合成的介孔催化剂显示出在各种苯乙烯(包括大体积和刚性的二苯乙烯及其异构体)的氢化中具有最大活性,达到约230000 h -1的TOF值。在聚合物模板存在下合成的其他介孔催化剂,但不添加 Si(OEt) 4,提供了反式环辛烯形成的选择性为 90-95%,与基于均相树枝状聚合物的催化剂相似。仅在 Si(OEt) 4存在下获得的微孔催化剂,同时充当锚定配体和模板的树枝状聚合物分子,在末端直链炔烃和共轭二烯的氢化中表现出最大活性,TOF 值高达 400000 h -1。在此,即使在 30 atm 的氢气压力下,对于末端炔烃和共轭二烯,烯烃的总选择性也达到
-
The first one-pot amidation of alkanes and cycloalkanes作者:Irena S. Akhrem、Dzul’etta V. Avetisyan、Lyudmila Afanas’eva、Sergei V. Vitt、Pavel V. Petrovskii、Alexander OrlinkovDOI:10.1016/j.tetlet.2007.12.070日期:2008.2Alkanes (or cycloalkanes) and CO in the presence of superelectrophilic systems CX4·2AlBr3 (X = Cl, Br) have been applied for the first time as equivalents of acylium salts in one-pot selective syntheses of amides from amines.
-
Colloidal and nanosized catalysts in organic synthesis: XV. Gas-phase hydrogenation of alkenes catalyzed by supported nickel nanoparticles作者:Yu. V. Popov、V. M. Mokhov、D. N. Nebykov、S. E. Latyshova、A. O. Panov、A. A. Dontsova、P. M. Shirkhanyan、K. V. ShcherbakovaDOI:10.1134/s1070363216120033日期:2016.12Gas-phase hydrogenation of alkenes and their derivatives, catalyzed by nickel nanoparticles supported on zeolite or silica gel support occurs at 150–250°С and an atmospheric hydrogen pressure and results in a high conversion. The selectivity of the hydrogenation depends on the amount of hydrogen: at a low diene (triene)–hydrogen ratio, selective hydrogenation of one multiple bond in the substrate is
表征谱图
-
氢谱1HNMR
-
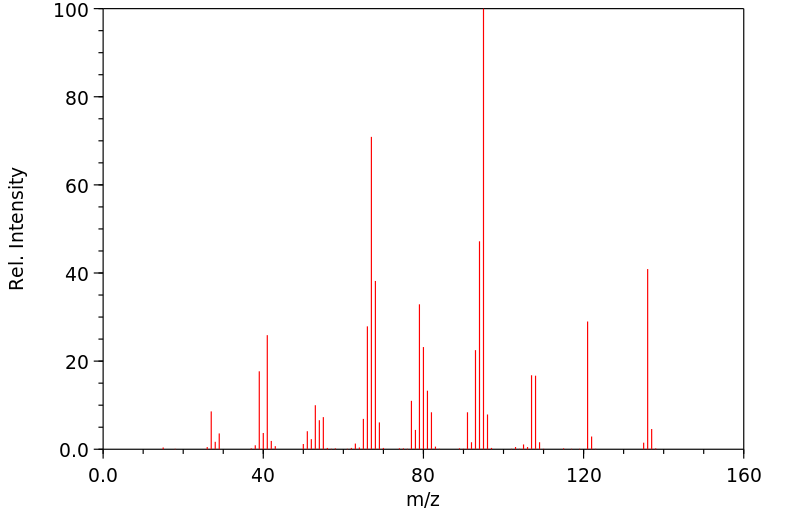
质谱MS
-
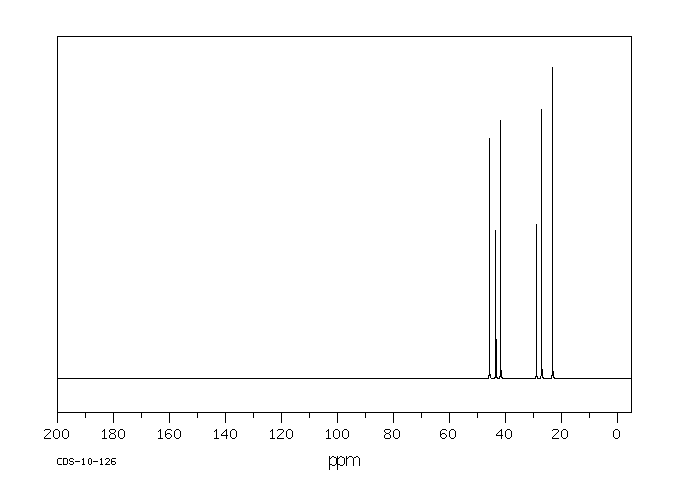
碳谱13CNMR
-
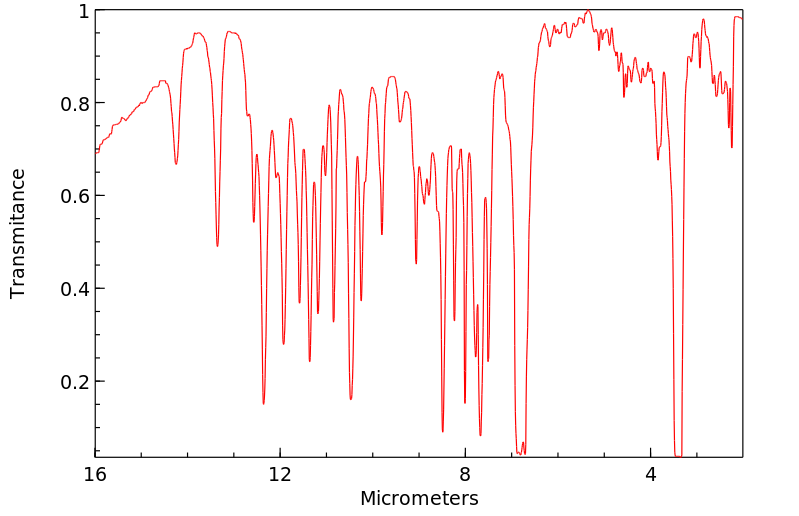
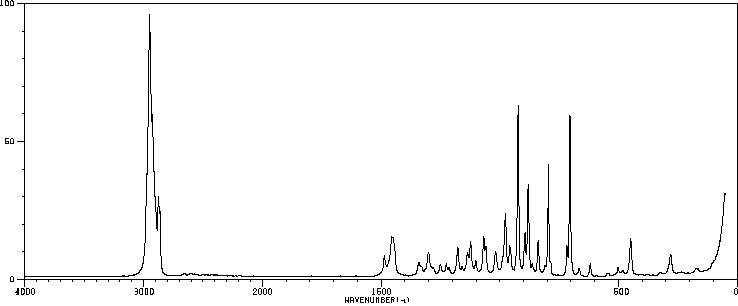
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸