2-氧代-4-苯基丁酸乙酯 | 64920-29-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:132 °C/2 mmHg (lit.)
-
密度:1.091 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:15
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S23,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
危险品运输编号:NONH for all modes of transport
-
海关编码:2918300090
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312+P330,P302+P352,P304+P340+P312,P305+P351+P338,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:存放在密封容器中,并放置在阴凉、干燥处。请确保储存位置远离氧化剂。
SDS
| Name: | Ethyl 2-oxo-4-phenylbutyrate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 64920-29-2 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 64920-29-2 | Ethyl 2-oxo-4-phenylbutyrate | 97% | 265-276-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 64920-29-2: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 241 deg C @ 760 mmH
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.091
Molecular Formula: C12H14O3
Molecular Weight: 206.0962
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 64920-29-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 2-oxo-4-phenylbutyrate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 64920-29-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 64920-29-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 64920-29-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:
- 用于有机合成中间体和药物中间体
- 作为有机中间体
- 是赖诺普利的中间体
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氧代-4-苯基丁酸 2-oxo-4-phenylbutyric acid 710-11-2 C10H10O3 178.188 —— ethyl 2-hydroxy-4-phenylbutanoate 93921-85-8 C12H16O3 208.257 氨基4-(2-乙基苯基)丁酸酯 ethyl 2-amino-4-phenylbutanoate 46460-24-6 C12H17NO2 207.272 2-羟基-4-苯基丁酸 2-hydroxy-4-phenylbutanoic acid 4263-93-8 C10H12O3 180.203 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— propyl 2-oxo-4-phenylbutyrate 83402-88-4 C13H16O3 220.268 —— Butyl 2-oxo-4-phenylbutanoate 83402-90-8 C14H18O3 234.295 —— Pentyl 2-oxo-4-phenylbutanoate 203263-70-1 C15H20O3 248.322 —— Propan-2-yl 2-oxo-4-phenylbutanoate 83402-89-5 C13H16O3 220.268 2-氧代-4-苯基丁酸甲酯 methyl 2-oxo-4-phenylbutanoate 83402-87-3 C11H12O3 192.214 —— 2-Methylpropyl 2-oxo-4-phenylbutanoate 83402-91-9 C14H18O3 234.295 —— 2,2-Dimethylpropyl 2-oxo-4-phenylbutanoate 203263-72-3 C15H20O3 248.322 —— tert-butyl 2-oxo-4-phenylbutanoate —— C14H18O3 234.295 2-氧代-4-苯基丁酸 2-oxo-4-phenylbutyric acid 710-11-2 C10H10O3 178.188 4-苯丁酸乙酯 ethyl 4-phenylbutyrate 10031-93-3 C12H16O2 192.258 —— ethyl 3-fluoro-2-oxo-4-phenylbutanoate 212712-69-1 C12H13FO3 224.232 3-氯-2-氧代-4-苯基丁酸乙酯 Ethyl 3-chloro-2-oxo-4-phenylbutanoate 145475-51-0 C12H13ClO3 240.686 3-溴-2-氧代-4-苯基丁酸乙酯 ethyl 3-bromo-2-oxo-4-phenylbutanoate 292858-05-0 C12H13BrO3 285.137 苄基2-氧代-4-苯基丁酸酯 benzyl 2-oxo-4-phenyl-butyrate 84688-29-9 C17H16O3 268.312 (S)-4-苯基-2-羟基丁酸乙酯 (S)-ethyl 2-hydroxy-4-phenylbutyrate 125639-64-7 C12H16O3 208.257 (R)-2-羟基-4-苯基丁酸乙酯 ethyl (R)-2-hydroxy-4-phenylbutyrate 90315-82-5 C12H16O3 208.257 —— ethyl 2-hydroxy-4-phenylbutanoate 93921-85-8 C12H16O3 208.257 (S)-2-氨基-4-苯基丁酸乙酯 L-homophenylalanine ethyl ester 46460-23-5 C12H17NO2 207.272 氨基4-(2-乙基苯基)丁酸酯 ethyl 2-amino-4-phenylbutanoate 46460-24-6 C12H17NO2 207.272 —— ethyl 2-phenethylpropenoate 27356-87-2 C13H16O2 204.269 —— (R)-(-)-Ethyl 3-hydroxy-4-phenylbutanoate 118856-11-4 C12H16O3 208.257 (RS)-2-羟基-4-苯基丁酸甲酯 (RS)-2-hydroxy-4-phenylbutanoic acid methyl ester 7226-82-6 C11H14O3 194.23 (R)-2-羟基-4-苯基丁酸 (R)-2-hydroxy4-phenylbutanoic acid 29678-81-7 C10H12O3 180.203 (S)-2-羟基-4-苯基丁酸 (S)-2-hydroxy-4-phenylbutanoic acid 115016-95-0 C10H12O3 180.203 —— phenyl 2-oxo-4-phenylbutyrate —— C16H14O3 254.285 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:N- [N-[(S)-1-乙氧基羰基-3-苯基丙基] -L-丙氨酰] -N-(茚满-2-基)甘氨酸(CV-3317)的设计与合成酶抑制剂。摘要:一种新的血管紧张素转换酶(ACE)抑制剂N-[N-[(S)-1-乙氧基碳基-3-苯基丙基]-L-丙氨酸]-N-(茚-2-基)甘氨酸(9a)是在对ACE抑制剂中C端氨基酸部分的结构要求进行广泛研究后发现的。本文描述了9a的合成及其绝对构型的确定。DOI:10.1248/cpb.34.2852
-
作为产物:描述:参考文献:名称:Synthesis and angiotensin converting enzyme inhibitory activity of 1,5-benzothiazepine and 1,5-benzoxazepine derivatives. I.摘要:新型血管紧张素转化酶(ACE)抑制剂的设计与合成,包括(R)-3-氨基-4-氧代-2, 3, 4, 5-四氢-1, 5-苯硫氮杂环-5-乙酸(7, 26, 33 和 37)以及(S)-3-氨基-4-氧代-2, 3, 4, 5-四氢-1, 5-苯氧氮杂环-5-乙酸(8 和 27),本文进行了描述。这些系列中的一些化合物在体外和体内显示出强烈的ACE抑制活性。同时讨论了结构-活性关系。DOI:10.1248/cpb.34.1128
文献信息
-
Methodology Development in Directed Evolution: Exploring Options when Applying Triple-Code Saturation Mutagenesis作者:Ge Qu、Richard Lonsdale、Peiyuan Yao、Guangyue Li、Beibei Liu、Manfred T. Reetz、Zhoutong SunDOI:10.1002/cbic.201700562日期:2018.2.2Directed evolution: Two strategies regarding the application of triple‐code saturation mutagenesis (TCSM) at multiresidue sites of the T. brockii alcohol dehydrogenase (TbSADH) by using distinct reduced amino‐acid alphabets are compared. By using prochiral tetrahydrofuran‐3‐one, highly R‐ and S‐selective variants are obtained with minimal screening. The origin of stereoselectivity is provided by molecular
-
A genomic search approach to identify carbonyl reductases in <i>Gluconobacter oxydans</i> for enantioselective reduction of ketones作者:Rong Chen、Xu Liu、Jinping Lin、Dongzhi WeiDOI:10.1080/09168451.2014.925775日期:2014.8.3
Abstract The versatile carbonyl reductases from Gluconobacter oxydans in the enantioselective reduction of ketones to the corresponding alcohols were exploited by genome search approach. All purified enzymes showed activities toward the tested ketoesters with different activities. In the reduction of 4-phenyl-2-butanone with in situ NAD(P)H regeneration system, (S)-alcohol was obtained with an e.e. of up to 100% catalyzed by Gox0644. Under the same experimental condition, all enzymes catalyzed ethyl 4-chloroacetoacetate to give chiral products with an excellent e.e. of up to 99%, except Gox0644. Gox2036 had a strict requirement for NADH as the cofactor and showed excellent enantiospecificity in the synthesis of ethyl (R)-4-chloro-3-hydroxybutanoate. For the reduction of ethyl 2-oxo-4-phenylbutyrate, excellent e.e. (>99%) and high conversion (93.1%) were obtained by Gox0525, whereas the other enzymes showed relatively lower e.e. and conversions. Among them, Gox2036 and Gox0525 showed potentials in the synthesis of chiral alcohols as useful biocatalysts.
摘要 通过基因组搜索方法,利用Gluconobacter oxydans中多功能醛酮还原酶对酮类化合物进行对映选择性还原,获得了一系列酶。所有纯化的酶对不同活性的酮酯表现出活性。在使用原位NAD(P)H再生系统还原4-苯基-2-丁酮时,Gox0644催化得到了e.e.高达100%的(S)-醇。在相同的实验条件下,除Gox0644外,所有酶均催化乙酸乙酯4-氯乙酮酸酯生成具有高达99%的优异e.e.的手性产物。Gox2036对NADH作为辅因子有严格要求,在合成乙酸(R)-4-氯-3-羟基丁酸乙酯中表现出优异的对映选择性。在还原乙酸2-氧代-4-苯基丁酸酯时,Gox0525获得了优异的e.e.(>99%)和高转化率(93.1%),而其他酶则表现出相对较低的e.e.和转化率。其中,Gox2036和Gox0525在合成手性醇作为有用的生物催化剂方面表现出潜力。
-
Efficient α-Methylenation of Carbonyl Compounds in Ionic Liquids at Room Temperature作者:J. Rodrigues、Juliana Vale、Daniel Zanchetta、Paulo MoranDOI:10.1055/s-0028-1087389日期:——The application of several 1-butyl-3-methylimidazolium (BMIM) salt ionic liquids as solvent in the α-methylenation of carbonyl compounds at room temperature is reported. The ionic liquid [BMIM][NTf2] gave a clean reaction in a short time and good yields of several α-methylene carbonyl compounds. This ionic liquid was reused without affecting the reaction rates or yields over seven runs.
-
Chiral Surfactant-Type Catalyst: Enantioselective Reduction of Long-Chain Aliphatic Ketoesters in Water作者:Zechao Lin、Jiahong Li、Qingfei Huang、Qiuya Huang、Qiwei Wang、Lei Tang、Deying Gong、Jun Yang、Jin Zhu、Jingen DengDOI:10.1021/acs.joc.5b00241日期:2015.5.1ligands were designed and synthesized. The rhodium complexes with the ligands were applied to the asymmetric transfer hydrogenation of broad range of long-chained aliphatic ketoesters in neat water. Quantitative conversion and excellent enantioselectivity (up to 99% ee) was observed for α-, β-, γ-, δ- and ε-ketoesters as well as for α- and β-acyloxyketone using chiral surfactant-type catalyst 2. The CH/π
-
Method for homogeneous enantioselective hydrogenation using catalytic ferrocenyl bis-phosphine complexes申请人:Degussa-Huls AG公开号:US06348620B1公开(公告)日:2002-02-19The present invention is relative to a method for the homogeneous, catalytic, enantioselective hydrogenation of compounds of the general formula (I) with the aid of compounds of the general formula (II) The use of the hydrogenated derivatives in organic synthesis.本发明涉及一种用于对一般式(I)化合物进行均相、催化、对映选择性氢化的方法,借助一般式(II)化合物。利用氢化衍生物在有机合成中的应用。
表征谱图
-
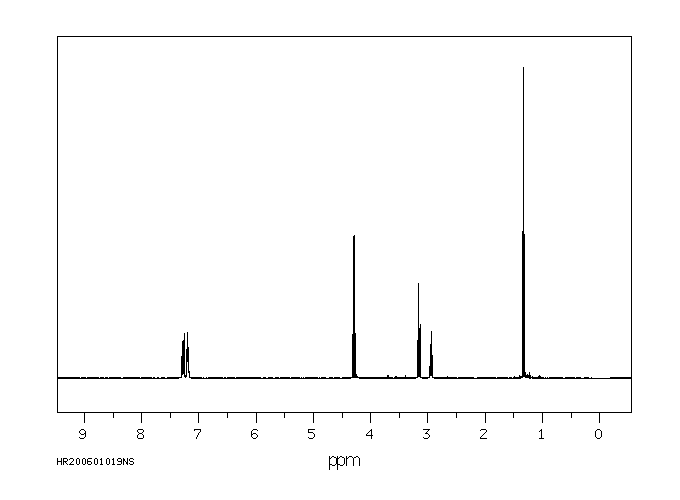
氢谱1HNMR
-
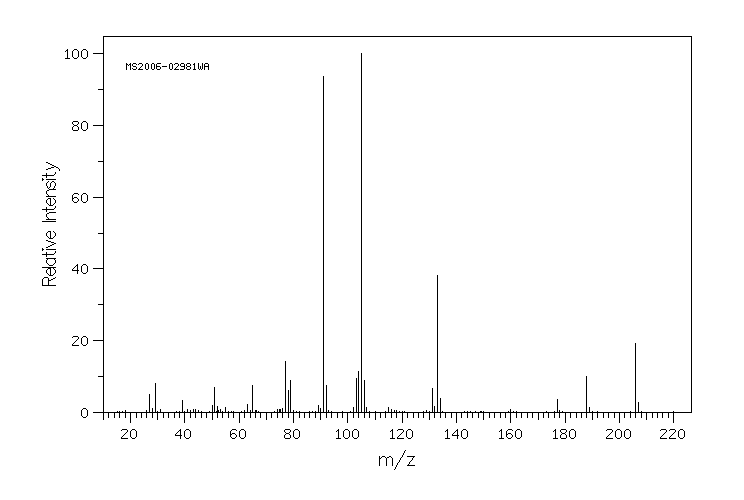
质谱MS
-
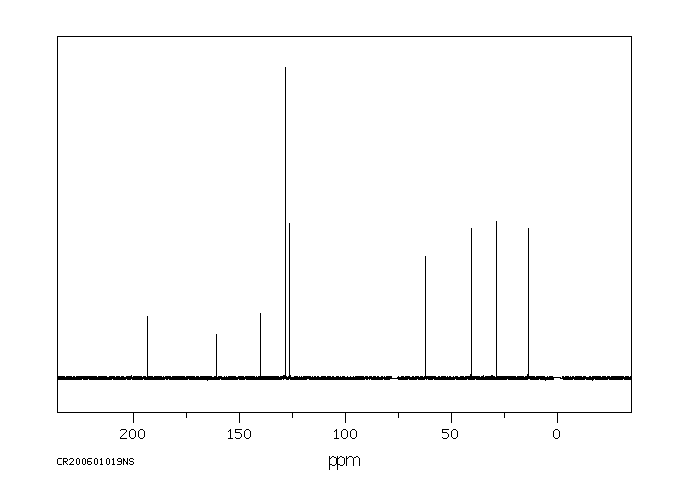
碳谱13CNMR
-
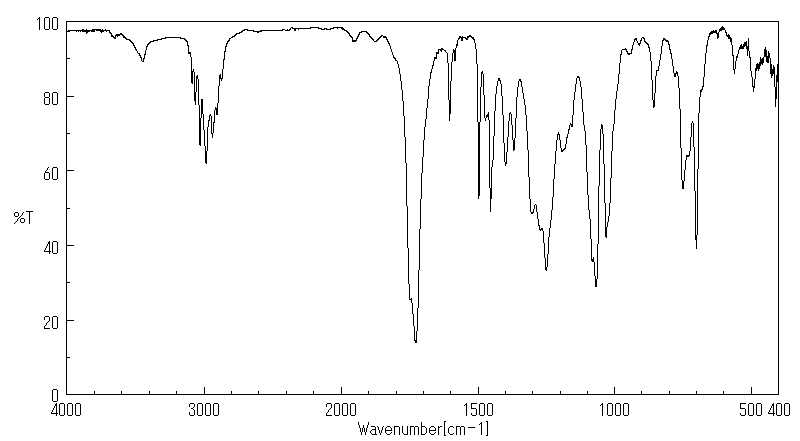
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息