(2E)-1-(2-羟基苯基)-3-苯基-2-丙烯-1-酮 | 888-12-0
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:88 °C
-
溶解度:可溶于氯仿、乙酸乙酯、甲醇
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
储存条件:| 室温 |
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-2'-hydroxy-chalcone 22029-75-0 C15H12O2 224.259 —— 2'-O-methoxymethylchalcone 40524-63-8 C17H16O3 268.312 —— 2'-O-isopropylchalcone —— C18H18O2 266.34 2'-羟基苯乙酮 o-hydroxyacetophenone 118-93-4 C8H8O2 136.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-2'-hydroxy-chalcone 22029-75-0 C15H12O2 224.259 —— 2',5'-dihydroxychalcone 52923-51-0 C15H12O3 240.258 —— (E)-1-(2-hydroxyphenyl)-3-phenylprop-2-en-1-one-2-d 55124-71-5 C15H12O2 225.251 —— 2'-O-methoxymethylchalcone 40524-63-8 C17H16O3 268.312 —— (E)-1-[2-[2-(dimethylamino)ethoxy]phenyl]-3-phenylprop-2-en-1-one —— C19H21NO2 295.4 —— trans-2'-acetoxychalcone 40524-64-9 C17H14O3 266.296 —— cis-2'-acetoxychalcone 138610-98-7 C17H14O3 266.296 —— 3',5'-dibromo-2'-hydroxy-trans-chalcone 15482-67-4 C15H10Br2O2 382.051 —— (2-Cinnamoylphenoxy)acetic acid 126861-85-6 C17H14O4 282.296 —— 2-[2-[(E)-3-phenylprop-2-enoyl]phenoxy]acetyl chloride 1027092-54-1 C17H13ClO3 300.741 —— (E)-1-(2-(benzyloxy)phenyl)-3-phenylprop-2-en-1-one 40524-65-0 C22H18O2 314.384 —— 2-cinnamylphenol 23925-16-8 C15H14O 210.276 —— Ethyl (2-cinnamoylphenoxy)acetate 144849-44-5 C19H18O4 310.35 —— 1-Oxo-1-(2-methansulfonyloxy-phenyl)-3-phenyl-propen-2 19334-31-7 C16H14O4S 302.351 —— (E)-2-(1-hydroxy-3-phenylallyl)phenol —— C15H14O2 226.275 —— aurone 37542-14-6 C15H10O2 222.243 —— aurone 582-04-7 C15H10O2 222.243 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:利用FeCl 3 ·6H 2 O-甲醇对2'-氨基和2'-羟基查耳酮进行新型一锅氧化环化反应。4-烷氧基-2-芳基喹啉和黄酮的合成摘要:通过在温和的条件下使用FeCl 3 ·6H 2 O-甲醇,对2'-氨基和2'-羟基查尔酮进行了简单,廉价且有效的氧化环化反应。已经研究了该方法用于合成2-(1,3-二苯基-1H-吡唑-4-基)-4-甲氧基喹啉。DOI:10.1016/j.tet.2007.06.051
-
作为产物:描述:1-乙酰氧基-2-碘苯 在 bis-triphenylphosphine-palladium(II) chloride 、 copper(l) iodide 、 对甲苯磺酸 、 三乙胺 作用下, 以 N,N-二甲基甲酰胺 、 苯 为溶剂, 反应 48.0h, 生成 (2E)-1-(2-羟基苯基)-3-苯基-2-丙烯-1-酮参考文献:名称:De, Mahuya; Majumdar, Dyuti P.; Kundu, Nitya G., Journal of the Indian Chemical Society, 1999, vol. 76, # 11-12, p. 665 - 674摘要:DOI:
-
作为试剂:描述:对甲基苯磺酰甲基异腈 在 (2E)-1-(2-羟基苯基)-3-苯基-2-丙烯-1-酮 、 silver(I) acetate 、 potassium carbonate 作用下, 以 乙醇 为溶剂, 以87 %的产率得到4-(4-methylbenzene-1-sulfonyl)-1-[(4-methylbenzene-1-sulfonyl)methyl]-1H-imidazol参考文献:名称:通过串联 [3 + 2] 环加成和分子内 C-O 偶联获得铬吡咯摘要:设计了一种温和、简洁的从 2'-羟基查尔酮合成色吡咯的方法。该反应通过 2'-羟基查耳酮和 1,3-亲偶极试剂的 C=C 键上的初始 [3 + 2] 环加成进行,该键是由异氰基乙酸乙酯和 AgOAc 反应原位生成的。然后与原位生成的吡咯的 -OH 基团和 C5-H 形成分子内 C-O 键,形成色并吡咯。DOI:10.1021/acs.joc.3c02479
文献信息
-
Asymmetric Cyclization of 2′-Hydroxychalcones to Flavanones: Catalysis by Chiral Brønsted Acids and Bases作者:Claudia Dittmer、Gerhard Raabe、Lukas HintermannDOI:10.1002/ejoc.200700682日期:2007.12the realization of an asymmetric flavanone synthesis by means of camphorsulfonic acid as chiral Bronsted acid catalysts were reinvestigated using accurate HPLC methods for quantification of enantiomer ratios. The previous claims of asymmetric induction were thus shown to be untenable. On the other hand, cinchona alkaloids serve as chiral Bronsted base mediators for the asymmetric cyclization of either2'-羟基查尔酮不对称环化为黄烷酮是类黄酮天然产物生物合成中的基本酶催化步骤,但对小分子催化剂的不对称催化造成了长期存在的问题。先前关于通过樟脑磺酸作为手性布朗斯台德酸催化剂实现不对称黄烷酮合成的声明使用准确的 HPLC 方法对对映异构体比率进行量化,重新研究。因此,先前关于不对称归纳的主张被证明是站不住脚的。另一方面,金鸡纳生物碱作为手性布朗斯台德碱介体用于 6'-取代的 2'-羟基查耳酮或 2',6'-二羟基查耳酮的不对称环化。例如,2',6'-二羟基-4,4'-二甲氧基查尔酮环化得到天然存在的柚皮素-4',7-二甲醚的 ee 高达 64%,转化率为 81%。催化剂显示产物的对映体过量对催化剂、溶剂和反应物浓度的显着依赖性。基于这些 2'-羟基查尔酮不对称环化为黄烷酮的成功实例,可以定义对活性更高的不对称催化剂的要求。(© Wiley-VCH Verlag GmbH & Co. KGaA,69451
-
Silica gel supported InBr3 and InCl3: new catalysts for the facile and rapid oxidation of 2′-hydroxychalcones and flavanones to their corresponding flavones under solvent free conditions作者:Naseem Ahmed、Hasrat Ali、Johan E. van LierDOI:10.1016/j.tetlet.2004.11.062日期:2005.1Silica gel supported InBr3 or InCl3 (15–20 mol %) were explored as a new solid-support catalysts for the facile and efficient oxidation, under solvent free conditions, of 2′-hydroxychalcones and flavanones to yield the corresponding flavones in >80% yield. The catalysts are easily prepared, stable, and efficient under mild reaction conditions.
-
Synthesis of Enones and Enals via Dehydrogenation of Saturated Ketones and Aldehydes作者:Gao-Fei Pan、Xue-Qing Zhu、Rui-Li Guo、Ya-Ru Gao、Yong-Qiang WangDOI:10.1002/adsc.201801058日期:2018.12.21substrate scope including various linear or cyclic saturated ketones and aldehydes. The protocol is ligand‐free, and molecular oxygen is used as the sole clean oxidant in the reaction. Due to mild reaction conditions, good functional group compatibility, and versatile utilities of enones and enals, the method can be applied in the late‐stage synthesis of natural products, pharmaceuticals and fine chemicals
-
Small Multitarget Molecules Incorporating the Enone Moiety作者:Thalia Liargkova、Nikolaos Eleftheriadis、Frank Dekker、Efstathia Voulgari、Constantinos Avgoustakis、Marina Sagnou、Barbara Mavroidi、Maria Pelecanou、Dimitra Hadjipavlou-LitinaDOI:10.3390/molecules24010199日期:——
Chalcones represent a class of small drug/druglike molecules with different and multitarget biological activities. Small multi-target drugs have attracted considerable interest in the last decade due their advantages in the treatment of complex and multifactorial diseases, since “one drug-one target” therapies have failed in many cases to demonstrate clinical efficacy. In this context, we designed and synthesized potential new small multi-target agents with lipoxygenase (LOX), acetyl cholinesterase (AChE) and lipid peroxidation inhibitory activities, as well as antioxidant activity based on 2-/4- hydroxy-chalcones and the bis-etherified bis-chalcone skeleton. Furthermore, the synthesized molecules were evaluated for their cytotoxicity. Simple chalcone b4 presents significant inhibitory activity against the 15-human LOX with an IC50 value 9.5 µM, interesting anti-AChE activity, and anti-lipid peroxidation behavior. Bis-etherified chalcone c12 is the most potent inhibitor of AChE within the bis-etherified bis-chalcones followed by c11. Bis-chalcones c11 and c12 were found to combine anti-LOX, anti-AchE, and anti-lipid peroxidation activities. It seems that the anti-lipid peroxidation activity supports the anti-LOX activity for the significantly active bis-chalcones. Our circular dichroism (CD) study identified two structures capable of interfering with the aggregation process of Aβ. Compounds c2 and c4 display additional protective actions against Alzheimer’s disease (AD) and add to the pleiotropic profile of the chalcone derivatives. Predicted results indicate that the majority of the compounds with the exception of c11 (144 Å) can cross the Blood Brain Barrier (BBB) and act in CNS. The results led us to propose new leads and to conclude that the presence of a double enone group supports better biological activities.
查耳酮类药物代表了一类具有多种和多重靶点生物活性的小分子药物或类药分子。在过去的十年里,小型多靶点药物因其能够治疗复杂和多因素疾病的优点而引起了相当大的兴趣,因为“一种药物一个靶点”的治疗方法在许多情况下未能显示出临床疗效。在这种情况下,我们设计并合成了具有潜在的新小型多靶点药物,具有脂氧合酶(LOX)、乙酰胆碱酯酶(AChE)和脂质过氧化抑制活性,以及基于2-/4-羟基查耳酮和双醚化双查耳酮骨架的抗氧化活性。此外,合成的分子还对其细胞毒性进行了评估。简单的查耳酮b4对15-人LOX具有显著的抑制活性,IC50值为9.5 µM,具有有趣的抗AChE活性和抗脂质过氧化行为。双醚化查耳酮c12是双醚化双查耳酮中对AChE最有效的抑制剂,其次是c11。发现双查耳酮c11和c12具有抗LOX、抗AchE和抗脂质过氧化活性。看来抗脂质过氧化活性支持显著活性的双查耳酮的抗LOX活性。我们的圆二色性(CD)研究发现两种结构能够干扰Aβ的聚集过程。化合物c2和c4显示了额外的保护作用,防止阿尔茨海默病(AD)的发生,并增加了查耳酮衍生物的多效性特征。预测结果表明,除了c11(144 Å)之外,大多数化合物能够穿越血脑屏障(BBB)并在中枢神经系统发挥作用。这些结果使我们提出了新的线索,并得出结论,存在一个双烯酮基团可以支持更好的生物活性。 -
NHC-Catalyzed Reaction of Enals with Hydroxy Chalcones: Diastereoselective Synthesis of Functionalized Coumarins作者:Anup Bhunia、Atanu Patra、Vedavati G. Puranik、Akkattu T. BijuDOI:10.1021/ol400562z日期:2013.4.5The N-heterocyclic carbene-catalyzed annulation of enals with 2′-hydroxy chalcones afford cyclopentane-fused coumarin derivatives with an excellent level of diastereocontrol. The reaction tolerates a broad range of functional groups; 25 examples are given, and a preliminary mechanistic investigation is provided.
表征谱图
-
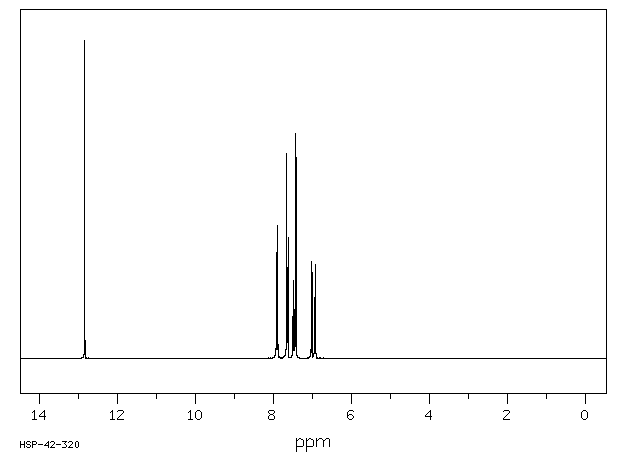
氢谱1HNMR
-
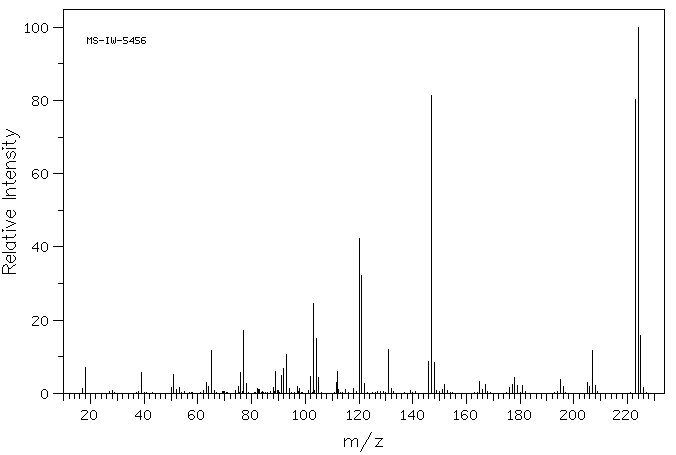
质谱MS
-
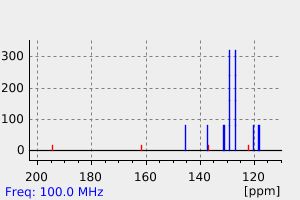
碳谱13CNMR
-
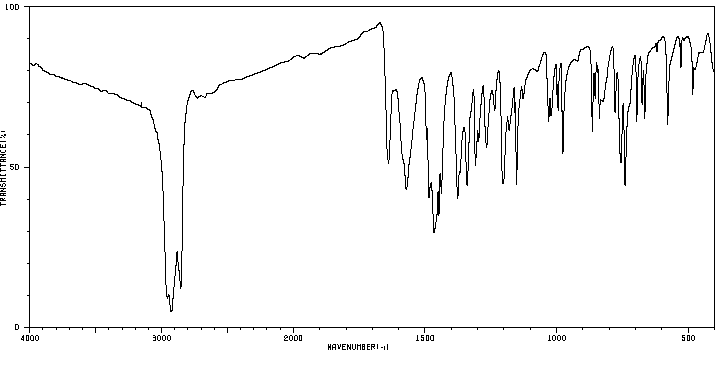
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息