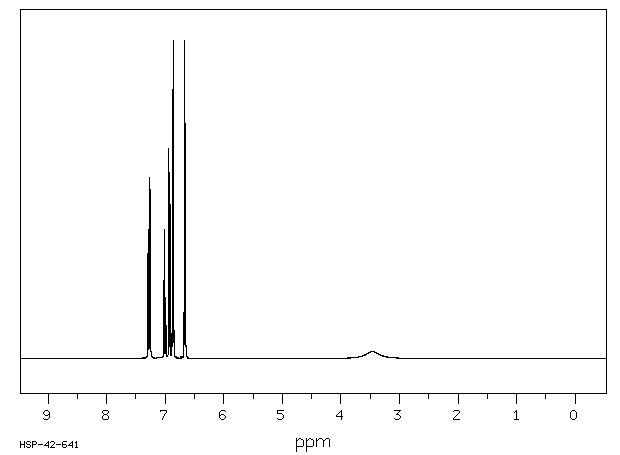
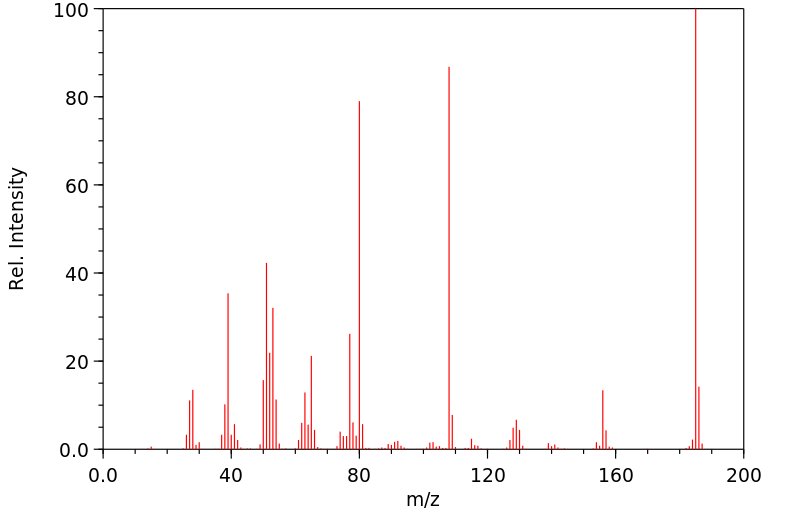
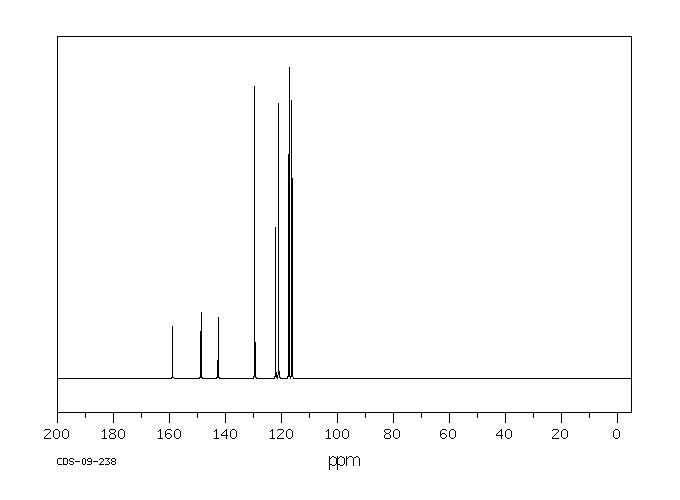
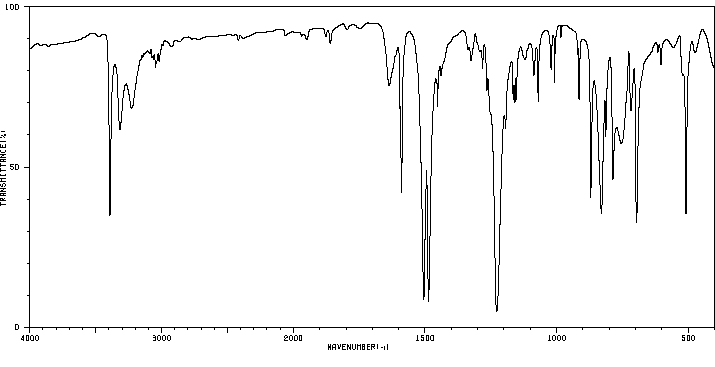
代谢
4-苯氧基苯胺已知的人类代谢物包括N-(4-苯氧基苯基)乙酰胺。
4-phenoxyaniline has known human metabolites that include N-(4-Phenoxyphenyl)acetamide.
来源:NORMAN Suspect List Exchange