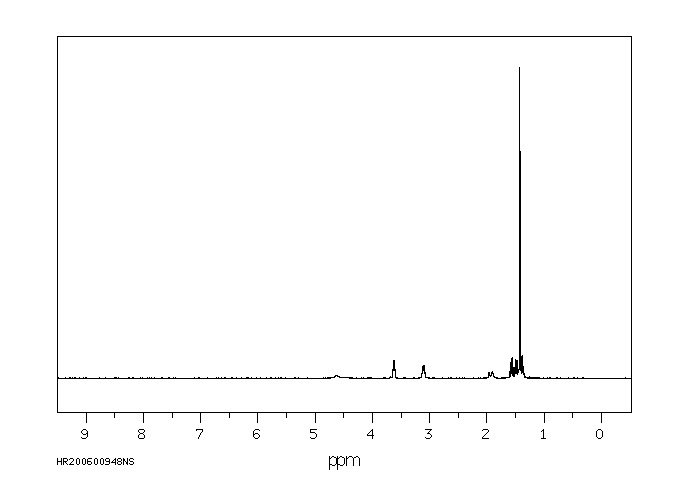
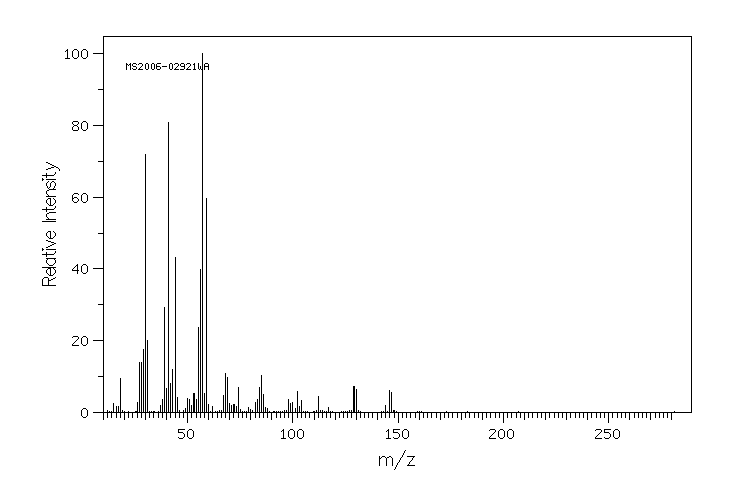
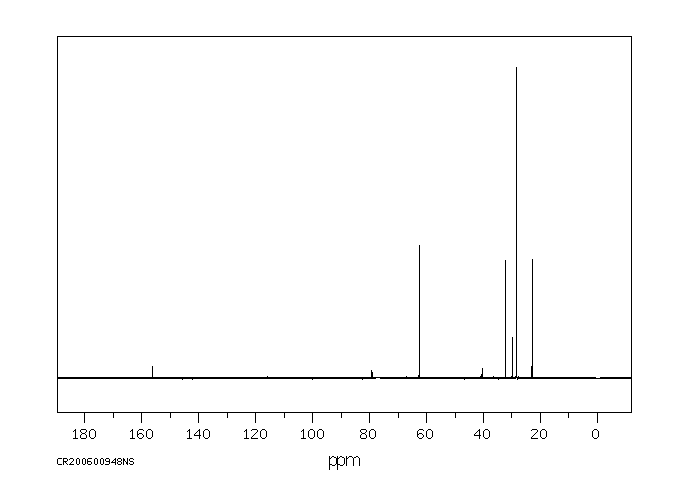
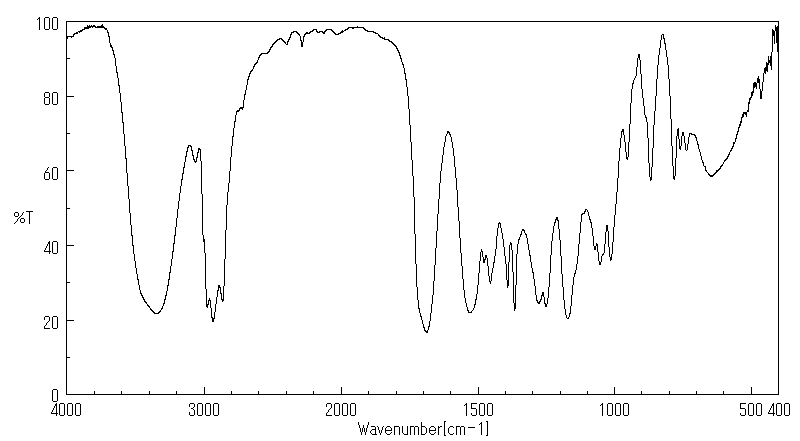
5-(N-叔丁氧羰基氨基)-1-戊醇 | 75178-90-4
中文名称
5-(N-叔丁氧羰基氨基)-1-戊醇
中文别名
N-Boc-5-氨基戊醇;TERT-BUTYLN-(5-羟基苯基)氨基甲酸叔丁酯;5-(叔丁氧羰氨基)-1-戊醇;5-(叔丁氧羰基-氨基)-1-戊醇;5-(Boc-氨基)-1-戊醇;N-(5-羟基戊基)氨基甲酸叔丁酯;(5-羟基戊基)氨基甲酸叔丁酯;5-(BOC-氨基)-1-戊醇
英文名称
5-(tert-butoxycarbonyl)amino-1-pentanol
英文别名
N-(tert-butyloxycarbonyl)-5-aminopentanol;tert-butyl (5-hydroxypentyl)carbamate;tert-butyl N-(5-hydroxypentyl)carbamate;tert‐butyl (5‐hydroxypentyl)carbamate;5-(Boc-amino)-1-pentanol
CAS
75178-90-4
化学式
C10H21NO3
mdl
——
分子量
203.282
InChiKey
DDGNGFVNTZJMMZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:318.4±25.0 °C(Predicted)
-
密度:1.00 g/mL at 20 °C(lit.)
-
闪点:>109°C
-
溶解度:可溶于氯仿、二氯甲烷、乙酸乙酯、甲醇
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:14
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.9
-
拓扑面积:58.6
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S23,S24/25
-
WGK Germany:3
-
海关编码:2924199090
-
危险性防范说明:P264,P270,P301+P312,P330
-
危险性描述:H302
-
储存条件:应将存放在充有干燥惰性气体的容器中,并置于阴凉、干燥处。避免接触湿气和水分。请远离氧化剂及水源。如有条件,建议冷藏保存。
SDS
5-(tert-Butoxycarbonylamino)-1-pentanol Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 5-(tert-Butoxycarbonylamino)-1-pentanol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 5-(tert-Butoxycarbonylamino)-1-pentanol
Percent: >97.0%(GC)
CAS Number: 75178-90-4
Synonyms: N-Boc-5-aminopentanol , tert-Butyl N-(5-Hydroxypentyl)carbamate , N-(5-
Hydroxypentyl)carbamic Acid tert-Butyl Ester
Chemical Formula: C10H21NO3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
5-(tert-Butoxycarbonylamino)-1-pentanol
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: clear
Color: Colorless - Almost colorless
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: 1.01
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Incompartible materials: oxidizing agents
5-(tert-Butoxycarbonylamino)-1-pentanol
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
5-(tert-Butoxycarbonylamino)-1-pentanol
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 5-(tert-Butoxycarbonylamino)-1-pentanol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 5-(tert-Butoxycarbonylamino)-1-pentanol
Percent: >97.0%(GC)
CAS Number: 75178-90-4
Synonyms: N-Boc-5-aminopentanol , tert-Butyl N-(5-Hydroxypentyl)carbamate , N-(5-
Hydroxypentyl)carbamic Acid tert-Butyl Ester
Chemical Formula: C10H21NO3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
5-(tert-Butoxycarbonylamino)-1-pentanol
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: clear
Color: Colorless - Almost colorless
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: 1.01
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Incompartible materials: oxidizing agents
5-(tert-Butoxycarbonylamino)-1-pentanol
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
5-(tert-Butoxycarbonylamino)-1-pentanol
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
5-(N-叔丁氧羰基氨基)-1-戊醇可以作为有机合成中间体和医药中间体,在实验室研发和化工生产过程中具有广泛应用。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 Boc-5-氨基戊酸 5-(N-tert-butyloxycarbonyl)aminopentanoic acid 27219-07-4 C10H19NO4 217.265 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(5-氨基戊基)氨基甲酸叔丁酯 5-tert-butoxycarbonylamino-1-aminopentane 51644-96-3 C10H22N2O2 202.297 —— 5-(methylamino)-N-Boc-pentanamine 1311458-36-2 C11H24N2O2 216.324 —— tert-butyl (5-oxopentyl)carbamate 94136-78-4 C10H19NO3 201.266 5-(T-boc-氨基)-1-戊基溴 tert-butyl (5-bromopentyl)carbamate 83948-54-3 C10H20BrNO2 266.178 —— tert-butyl (5-iodopentyl)carbamate 262278-05-7 C10H20INO2 313.179 —— tert-butyl (5-fluoropentyl)carbamate 1445406-54-1 C10H20FNO2 205.273 4-氯丁基氨基甲酸叔丁酯 tert-butyl (4-chlorobutyl)carbamate 95388-79-7 C9H18ClNO2 207.7 N-Boc-5-重氮基戊-1-胺 (5-azido-pentyl)carbamic acid tert-butyl ester 129392-86-5 C10H20N4O2 228.294 —— tert-butyl (5-((5-azidopentyl)oxy)pentyl)carbamate —— C15H30N4O3 314.428 —— tert-butyl (5-hydroxypentyl)(methyl)carbamate 1373210-02-6 C11H23NO3 217.309 —— (2E)-7-{[(tert-butoxy)carbonyl]amino}hept-2-enoic acid 1040031-88-6 C12H21NO4 243.303 —— 5-[(2-Methylpropan-2-yl)oxycarbonylamino]pentyl 2-cyanoacetate 1309612-67-6 C13H22N2O4 270.329 N-BOC-3-氯丙基胺 tert-butyl N-(3-chloropropyl)carbamate 116861-31-5 C8H16ClNO2 193.674 5-boc-氨基戊基甲烷磺酸酯 5-((tert-butoxycarbonyl)amino)pentyl methanesulfonate 142342-55-0 C11H23NO5S 281.373 甲基(5-氧代戊基)氨基甲酸叔丁酯 tert-butyl methyl(5-oxopentyl)carbamate 1620280-47-8 C11H21NO3 215.293 —— 2-Methyl-acrylic acid 5-tert-butoxycarbonylamino-pentyl ester 204319-95-9 C14H25NO4 271.357 —— 5-N-tert-butoxycarbonylaminopentyl (E,E)-2',4'-hexadienoate 640286-85-7 C16H27NO4 297.395 —— 4-((tert-butoxycarbonyl)amino)butyl methanesulfonate 174626-25-6 C10H21NO5S 267.346 —— tert-butyl (5-((tert-butyldimethylsilyl)oxy)pentyl)carbamate 500143-73-7 C16H35NO3Si 317.544 2,2,2-三氯乙基N-(5-氨基戊基)氨基甲酸酯 2,2,2-Trichloroethyl (5-aminopentyl)carbamate 139408-45-0 C8H15Cl3N2O2 277.578 —— tert-butyl [5-[(Benzyloxy)amino]pentyl]carbamate 153492-09-2 C17H28N2O3 308.421 —— benzyl N-[5-[(2-methylpropan-2-yl)oxycarbonylamino]pentyl]carbamate 181780-37-0 C18H28N2O4 336.431 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:双重烟酰胺磷酸核糖基转移酶和表皮生长因子受体抑制剂治疗癌症摘要:在现代抗癌治疗中,多靶点药物已成为一种有前途的治疗方式。利用NAMPT和EGFR抑制的协同作用,我们开发了首个可用作NAMPT和EGFR双重抑制剂的化合物。在CHS828和厄洛替尼的基础上,通过药效基团的合并,成功设计并合成了一系列杂交分子。在合成的化合物中,化合物28表现出良好的NAMPT和EGFR抑制作用,并具有出色的体外抗增殖活性。化合物28是不含Michael受体的新型化学型,可强烈抑制几种癌细胞系的增殖,包括具有EGFR L858R / T790M的H1975非小细胞肺癌细胞突变。更重要的是,它在人NSCLC(H1975)异种移植裸鼠模型中具有显着的体内抗肿瘤功效。这项研究为开发新型抗肿瘤药物和有价值的药理探针提供了有希望的线索,这些药物可用于评估单一抑制剂对NAMPT和EGFR途径的双重抑制作用。DOI:10.1016/j.ejmech.2020.113022
-
作为产物:描述:1-Boc-2-哌啶酮 在 Cp*RuCl{(C6H5)2P-(CH2)2NH2-κ2-P,N} 、 potassium tert-butylate 、 氢气 作用下, 以 叔丁醇 为溶剂, 80.0 ℃ 、3.0 MPa 条件下, 反应 24.0h, 以99%的产率得到5-(N-叔丁氧羰基氨基)-1-戊醇参考文献:名称:双官能[Cp * Ru(PN)]配合物催化的N-酰基氨基甲酸酯和N-酰基磺酰胺的加氢反应摘要:Cp 1的唤醒:双功能配合物1促进了与具有比酮,环氧化物和酰亚胺少的亲电子碳原子的底物的相互作用。该标题反应适用于将埃文斯的不对称烷基化产物还原为手性醇,以及良好的手性恶唑烷酮助剂的回收率。EWG =吸电子基团。DOI:10.1002/anie.200805307
-
作为试剂:描述:5-氨基-1-戊醇 、 二碳酸二叔丁酯 在 ice 、 氯仿 、 碳酸氢钠 、 水 、 magnesium sulfate 、 5-(N-叔丁氧羰基氨基)-1-戊醇 作用下, 以 二氯甲烷 为溶剂, 反应 18.0h, 生成 5-(N-叔丁氧羰基氨基)-1-戊醇参考文献:名称:MICROPHASE-SEPARATED STRUCTURE MEMBRANE AND PROCESS FOR PRODUCING THE SAME摘要:提供的是一种微相分离结构膜,其中包括一种嵌段共聚物,其中一种亲水性聚合物组分和一种疏水性聚合物组分通过具有反应性基团、电子受体或电子给体或染料的结构单元相互耦合。在微相分离结构膜中,由亲水性聚合物组分组成的圆柱体结构位于由疏水性聚合物组分组成的基质中,并且沿垂直于膜表面的方向定向,具有反应性基团、电子受体或电子给体或染料的结构单元位于基质和圆柱体结构之间。公开号:US20100210742A1
文献信息
-
1-Amino 1H-imidazoquinolines申请人:Griesgraber W. George公开号:US20050054640A1公开(公告)日:2005-03-101-Amino 1H-imidazoquinoline compounds, pharmaceutical compositions containing the compounds, intermediates, and methods of making and methods of use of these compounds as immunomodulators, for modulating cytokine biosynthesis in animals and in the treatment of diseases including viral and neoplastic diseases are disclosed.
-
一种用于治疗肿瘤的黄酮衍生物及其应用申请人:四川福生源科技有限公司公开号:CN111620920A公开(公告)日:2020-09-04
-
IRAK DEGRADERS AND USES THEREOF申请人:Kymera Therapeutics, Inc.公开号:US20190192668A1公开(公告)日:2019-06-27The present invention provides compounds, compositions thereof, and methods of using the same.本发明提供了化合物、其组合物以及使用这些化合物的方法。
-
[EN] MACROCYCLIC COMPOUNDS FOR MODULATING IL-17<br/>[FR] COMPOSÉS MACROCYCLIQUES POUR UNE MODULATION D'IL-17申请人:ENSEMBLE THERAPEUTICS CORP公开号:WO2013116682A1公开(公告)日:2013-08-08The invention relates generally to macrocyclic compounds of formula I and their therapeutic use. More particularly, the invention relates to macrocyclic compounds that modulate the activity of IL-17 and/or are useful in the treatment of medical conditions, such as inflammatory diseases and other IL-17-associated disorders.这项发明通常涉及公式I的大环化合物及其治疗用途。更具体地,该发明涉及调节IL-17活性的大环化合物,或者用于治疗炎症性疾病和其他与IL-17相关的疾病的大环化合物。
-
[EN] TETRAHYDROPYRIDOPYRIMIDINES AND TETRAHYDROPYRIDOPYRIDINES AS INHIBITORS OF HBSAG (HBV SURFACE ANTIGEN) AND HBV DNA PRODUCTION FOR THE TREATMENT OF HEPATITIS B VIRUS INFECTIONS<br/>[FR] TÉTRAHYDROPYRIDOPYRIMIDINES ET TÉTRAHYDROPYRIDOPYRIDINES COMME INHIBITEURS D'AG HBS (ANTIGÈNE DE SURFACE DU VIRUS DE L'HÉPATITE B) ET PRODUCTION D'ADN DE VHB POUR LE TRAITEMENT D'INFECTIONS PAR LE VIRUS DE L'HÉPATITE B申请人:HOFFMANN LA ROCHE公开号:WO2016177655A1公开(公告)日:2016-11-10The present invention provides tetrahydropyridopyrimidines and tetrahydropyridopyridines having the general formula (I) wherein R1, R2, U, W, X, Y and Z are as described herein, as inhibitors of HBsAg (HBV surface antigen) and HBV DNA production for the treatment and prophylaxis of hepatitis B virus infections.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸