N-茴香酰胺 | 7472-54-0
中文名称
N-茴香酰胺
中文别名
N-(4-甲氧苯基)苯甲酰胺;对苯茴香胺;4'-甲氧基苯酰替苯胺;N-苯甲酰对茴香胺
英文名称
N-(p-methoxyphenyl)benzamide
英文别名
N-(4-methoxyphenyl)benzamide;4'-methoxybenzanilide;p-benzanisidide
CAS
7472-54-0
化学式
C14H13NO2
mdl
MFCD00025788
分子量
227.263
InChiKey
KEEBHMMBUBEEOV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:155°C
-
沸点:290.2±23.0 °C(Predicted)
-
密度:1.31 g/cm3
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
海关编码:2924299090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:密封在阴凉干燥环境中。
SDS
p-Benzanisidide Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: p-Benzanisidide
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): p-Benzanisidide
Percent: >98.0%(GC)(T)
CAS Number: 7472-54-0
Synonyms: N-Benzoyl-p-anisidine , 4'-Methoxybenzanilide , N-(4-Methoxyphenyl)benzamide
Chemical Formula: C14H13NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
p-Benzanisidide
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Pale yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:155 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
p-Benzanisidide
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
p-Benzanisidide
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: p-Benzanisidide
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): p-Benzanisidide
Percent: >98.0%(GC)(T)
CAS Number: 7472-54-0
Synonyms: N-Benzoyl-p-anisidine , 4'-Methoxybenzanilide , N-(4-Methoxyphenyl)benzamide
Chemical Formula: C14H13NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
p-Benzanisidide
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Pale yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:155 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
p-Benzanisidide
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
p-Benzanisidide
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苯甲酰替苯胺 N-phenyl benzoyl amide 93-98-1 C13H11NO 197.236 —— N-(4-methoxyphenyl)-N-methyl-benzamide 33675-68-2 C15H15NO2 241.29 2-碘-N-(4-甲氧基苯基)苯甲酰胺 2-iodo-N-(4-methoxyphenyl)benzamide 36684-49-8 C14H12INO2 353.159 2-氟-N-(4-甲氧基苯基)苯甲酰胺 2-fluoro-N-(4-methoxyphenyl)benzamide 212209-96-6 C14H12FNO2 245.253 2-溴-N-(4-甲氧基苯基)苯甲酰胺 2-bromo-N-(4-methoxyphenyl)benzamide 55204-50-7 C14H12BrNO2 306.159 2-巯基-N-(对甲氧基苯基)苯甲酰胺 2-mercapto-N-(p-methoxyphenyl)benzamide 92199-75-2 C14H13NO2S 259.329 N-[4-(三氟甲基)苯基]苯甲酰胺 N-(4-(trifluoromethyl)phenyl)benzamide 350-98-1 C14H10F3NO 265.235 N-甲基-N-苯基苯甲酰胺 N-methyl-N-phenyl-benzamide 1934-92-5 C14H13NO 211.263 N-(4-甲氧基苯基)苯硫代甲酰胺 N-(4-methoxyphenyl)benzothioamide 5310-26-9 C14H13NOS 243.329 N-烯丙基-N-(4-甲氧基苯基)苯甲酰胺 N-allyl-N-(4-methoxyphenyl)benzamide 71954-45-5 C17H17NO2 267.327 4-甲氧基-N,N-二苄基苯胺 N,N-dibenzyl-4-anisidine 18613-55-3 C21H21NO 303.404 N-苯甲酰苯基羟胺 N-phenylbenzohydroxamic acid 304-88-1 C13H11NO2 213.236 —— 2-(4-methyloxyphenylamino)-2-phenylacetonitrile 32323-74-3 C15H14N2O 238.289 2-(4-甲氧基苯基)异吲哚-1,3-二酮 2-(4-methoxyphenyl)isoindoline-1,3-dione 2142-04-3 C15H11NO3 253.257 —— N-(4-tert-butyldimethylsiloxybenzyl)-N-(4-methoxyphenyl)benzamide 1341219-55-3 C27H33NO3Si 447.649 —— N-(4-hydroxy-2-methoxybenzyl)-p-benzanisidide 1341219-51-9 C22H21NO4 363.413 4'-甲氧基甲酰苯胺 4-methoxyformanilide 5470-34-8 C8H9NO2 151.165 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(N-苯甲酰基氨基)苯酚 p-benzoylaminophenol 15457-50-8 C13H11NO2 213.236 N-苯甲酰替苯胺 N-phenyl benzoyl amide 93-98-1 C13H11NO 197.236 —— N-(4-methoxyphenyl)-N-methyl-benzamide 33675-68-2 C15H15NO2 241.29 N-苄基-4-甲氧基苯胺 N-benzyl-p-anisidine 17377-95-6 C14H15NO 213.279 N-(4-甲氧基苯基)-N-苯基苯甲酰胺 N-(4-methoxyphenyl)-N-phenylbenzamide 73333-81-0 C20H17NO2 303.36 —— 4-(benzoylamino)phenyl methanesulfonate 73153-59-0 C14H13NO4S 291.328 N-[4-(三氟甲基)苯基]苯甲酰胺 N-(4-(trifluoromethyl)phenyl)benzamide 350-98-1 C14H10F3NO 265.235 N-(4-甲氧基苯基)苯硫代甲酰胺 N-(4-methoxyphenyl)benzothioamide 5310-26-9 C14H13NOS 243.329 N-烯丙基-N-(4-甲氧基苯基)苯甲酰胺 N-allyl-N-(4-methoxyphenyl)benzamide 71954-45-5 C17H17NO2 267.327 —— 2-acetamido-N-(4-methoxyphenyl)benzamide 59525-22-3 C16H16N2O3 284.315 —— N-Benzoyl-N-(4-methoxyphenyl)glycine 28794-25-4 C16H15NO4 285.299 N-(4-甲氧基-2-硝基苯基)苯甲酰胺 N-(4-methoxy-2-nitrophenyl)benzamide 38259-63-1 C14H12N2O4 272.26 苯甲基苯胺 N-Benzylaniline 103-32-2 C13H13N 183.253 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:铜催化轻度硝化保护苯胺摘要:通过使用一当量的硝酸作为硝化剂,已开发出一种实用的铜催化的被保护的苯胺直接硝化方法。该程序的特点是反应条件温和,结构范围广(就N保护基和芳烃取代而言)以及高官能团耐受性。用两当量的硝酸进行消解也是可行的。DOI:10.1002/chem.201404000
-
作为产物:描述:参考文献:名称:钯催化的醛亚胺氧化成酰胺摘要:通过亚胺的直接氧化合成酰胺的方法鲜有报道。在这里,我们报告了一种通过瓦克型反应使用廉价的叔丁基氢过氧化物水溶液作为氧化剂将亚胺催化氧化为酰胺衍生物的有效方法。该方法实用方便且显示出高官能团耐受性,允许各种亚胺以中等至良好的产率转化为相应的酰胺衍生物。DOI:10.1055/s-0037-1610653
文献信息
-
一种利用嘧啶类缩合剂点击构建酰胺键的方法及其在酰胺和多肽合成中的应用申请人:兰州大学公开号:CN112851536B公开(公告)日:2022-02-25
-
An efficient mechanochemical synthesis of amides and dipeptides using 2,4,6-trichloro-1,3,5-triazine and PPh<sub>3</sub>作者:Chuthamat Duangkamol、Subin Jaita、Sirilak Wangngae、Wong Phakhodee、Mookda PattarawarapanDOI:10.1039/c5ra10127a日期:——mechanochemical synthesis of amides from carboxylic acids has been developed through an in situ acid activation with 2,4,6-trichloro-1,3,5-triazine and a catalytic amount of PPh3. Under room temperature solvent-drop grinding of the reactants in the presence of an inorganic base, a variety of carboxylic acids including aromatic acids, aliphatic acids, and N-protected α-amino acids undergo amidation to afford
-
Mechanism of Thio Acid/Azide Amidation作者:Robert V. Kolakowski、Ning Shangguan、Ronald R. Sauers、Lawrence J. WilliamsDOI:10.1021/ja057533y日期:2006.5.1A combined experimental and computational mechanistic study of amide formation from thio acids and azides is described. The data support two distinct mechanistic pathways dependent on the electronic character of the azide component. Relatively electron-rich azides undergo bimolecular coupling with thiocarboxylates via an anion-accelerated [3+2] cycloaddition to give a thiatriazoline. Highly electron-poor描述了从硫代酸和叠氮化物形成酰胺的联合实验和计算机制研究。数据支持两种不同的机械途径,这取决于叠氮化物组分的电子特性。相对富电子的叠氮化物通过阴离子加速的 [3+2] 环加成与硫代羧酸盐进行双分子偶联,得到噻三唑啉。高度缺电子的叠氮化物通过叠氮化物的末端氮与硫代羧酸盐的硫的双分子结合形成线性加合物。该中间体的环化得到噻三唑啉。发现分解为酰胺是通过中性噻三唑啉中间体的逆 [3+2] 环加成反应进行的。计算分析(DFT,6-31+G(d)) 确定了两类叠氮化物与硫代酸进行 [3+2] 环加成生成噻三唑啉中间体的途径,尽管这些途径的能量高于硫代羧酸酰胺化。这些研究还确定,缺电子叠氮化物的反应曲线可归因于先有捕获机制,然后是分子内酰化。
-
Direct Amidation of Esters by Ball Milling**作者:William I. Nicholson、Fabien Barreteau、Jamie A. Leitch、Riley Payne、Ian Priestley、Edouard Godineau、Claudio Battilocchio、Duncan L. BrowneDOI:10.1002/anie.202106412日期:2021.9.27The direct mechanochemical amidation of esters by ball milling is described. The operationally simple procedure requires an ester, an amine, and substoichiometric KOtBu and was used to prepare a large and diverse library of 78 amide structures with modest to excellent efficiency. Heteroaromatic and heterocyclic components are specifically shown to be amenable to this mechanochemical protocol. This
-
PCl<sub>3</sub>-mediated transesterification and aminolysis of <i>tert</i>-butyl esters via acid chloride formation作者:Xiaofang Wu、Lei Zhou、Fangshao Li、Jing XiaoDOI:10.1177/1747519820987530日期:2021.5A PCl3-mediated conversion of tert-butyl esters into esters and amides in one-pot under air is developed. This novel protocol is highlighted by the synthesis of skeletons of bioactive molecules and gram-scale reactions. Mechanistic studies revealed that this transformation involves the formation of an acid chloride in situ, which is followed by reactions with alcohols or amines to afford the desired
表征谱图
-
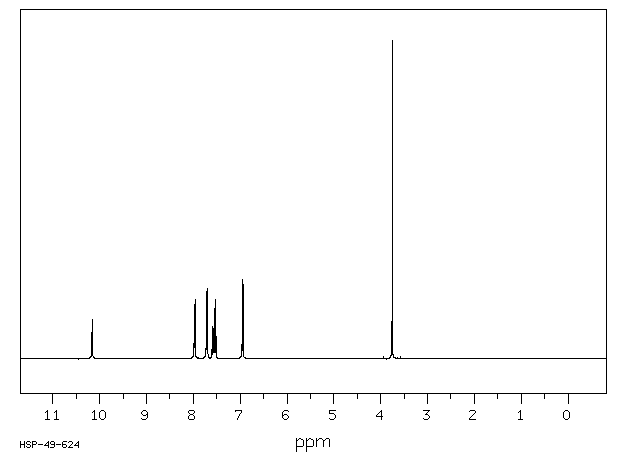
氢谱1HNMR
-
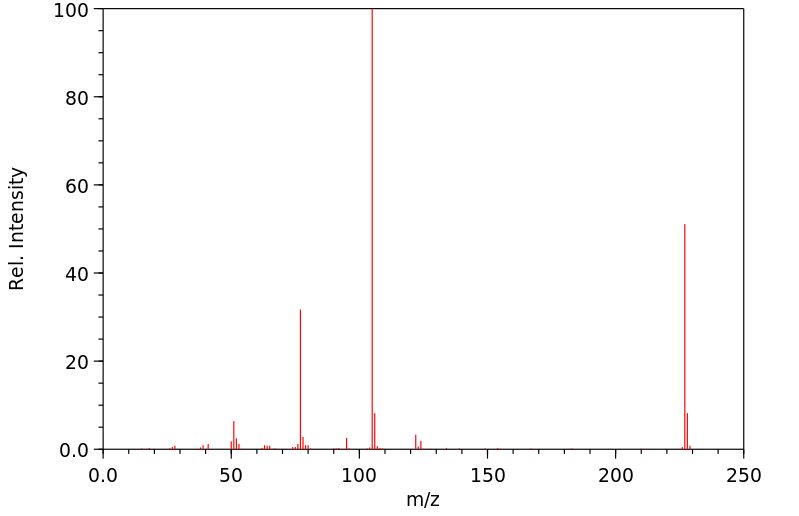
质谱MS
-
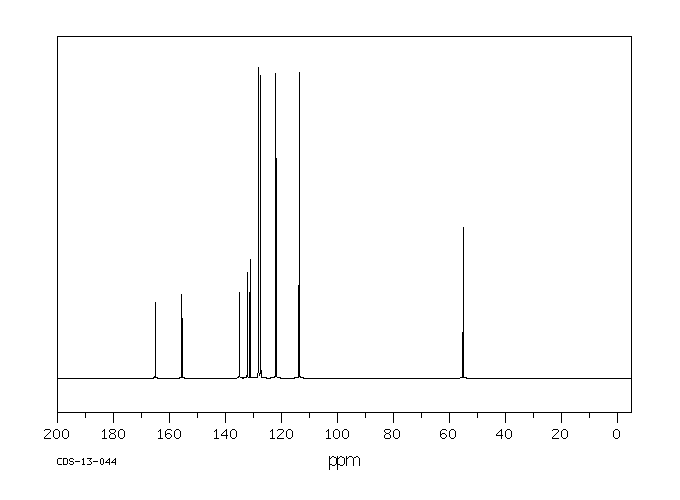
碳谱13CNMR
-
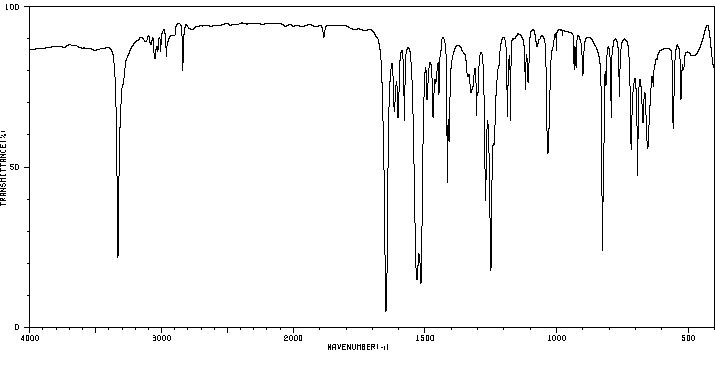
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫