反-5-癸烯 | 7433-56-9
中文名称
反-5-癸烯
中文别名
5-癸烯;反-1,2-二丁基乙烯;反式-5-癸烯
英文名称
(E)-5-decene
英文别名
trans-5-decene;(E)-dec-5-ene;5-decene;trans-dec-5-ene;dec-5-ene
CAS
7433-56-9
化学式
C10H20
mdl
——
分子量
140.269
InChiKey
UURSXESKOOOTOV-MDZDMXLPSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-73°C
-
沸点:170 °C/750 mmHg (lit.)
-
密度:0.74 g/mL at 25 °C (lit.)
-
闪点:115 °F
-
介电常数:2.0299999999999998
-
保留指数:992;992;992;992;1000;1000;993;984;992;1000
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:10
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品标志:Xi
-
安全说明:S16,S26,S36/37/39
-
危险类别码:R10
-
WGK Germany:3
-
危险品运输编号:UN 3295 3/PG 3
-
RTECS号:HE2080000
-
包装等级:III
-
危险类别:3
-
储存条件:密封在阴凉干燥的环境中。
SDS
| Name: | Trans-5-Decene 99+% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 7433-56-9 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7433-56-9 | Trans-5-Decene | >99 | 231-080-7 |
Risk Phrases: 10
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable.
Potential Health Effects
Eye:
May cause eye irritation. May cause chemical conjunctivitis and corneal damage.
Skin:
May cause irritation and dermatitis. May cause cyanosis of the extremities.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. Ingestion of large amounts may cause CNS depression.
Inhalation:
The toxicological properties of this substance have not been fully investigated. Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation. May cause burning sensation in the chest.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Water may be ineffective. Material is lighter than water and a fire may be spread by the use of water. Containers may explode in the heat of a fire.
Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 7433-56-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: aromatic odor
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 170 deg C @ 750.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 46 deg C ( 114.80 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .7400g/cm3
Molecular Formula: C10H20
Molecular Weight: 140.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7433-56-9: HE2080000 LD50/LC50:
Not available.
Carcinogenicity:
Trans-5-Decene - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
R 10 Flammable.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 7433-56-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 7433-56-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 7433-56-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 顺式-5-癸烯 cis-5-decene 7433-78-5 C10H20 140.269 5-癸烯 5-decene 19689-19-1 C10H20 140.269 顺-4-壬烯 (Z)-4-nonene 10405-84-2 C9H18 126.242 1-丁基癸烯 1-butyldecene 4084-07-5 C14H28 196.376 1-己烯 1-hexene 592-41-6 C6H12 84.1613 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (5E)-5-十二碳烯 (E)-dodec-5-ene 7206-16-8 C12H24 168.323 —— trans-2-methyl-3-octene 52937-36-7 C9H18 126.242 —— 5-tetradecene 4084-07-5 C14H28 196.376 1-丁基癸烯 1-butyldecene 4084-07-5 C14H28 196.376 —— 2-methyl-non-4t-ene 28665-55-6 C10H20 140.269 (9E)-9-十八碳烯 trans-9-octadecene 7206-25-9 C18H36 252.484 反-5-癸烯醇 (E)-5-decen-1-ol 56578-18-8 C10H20O 156.268 1-己烯 1-hexene 592-41-6 C6H12 84.1613 —— (E)-1-chloro-2-heptene 68703-33-3 C7H13Cl 132.633 —— (E)-2,2-dimethyloct-3-ene 62187-13-7 C10H20 140.269 反式-2-甲基-1,3-辛二烯 (E)-2-methylocta-1,3-diene 67350-81-6 C9H16 124.226 9,10-十四碳烯醇 (E)-9-tetradecen-1-ol 52957-16-1 C14H28O 212.376 —— 14-chloro-tetradec-5-ene 62706-15-4 C14H27Cl 230.821 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:内烯烃与叔胺作为氢供体在碳载贵金属上的氢转移反应†摘要:在碳载体上存在贵金属的情况下,观察到与叔胺的氢化物转移反应。以前已经在三乙胺的存在下以活化的烯丙基型碳-碳双键(含羰基衍生物)的形式记录了氢化物转移(共轭还原)。所提出的机理是氢化物转移反应,其中金属充当氢化物-金属亚胺鎓加合物形成的反应伙伴。在这项工作中首次观察到未活化的内部双键化合物(例如油酸甲酯和反式-5-癸烯)作为底物的饱和状态。预还原的催化剂样品显示出高活性。在Pd / C,Pt / C和铑/碳的存在下部分到完全转化在140℃下,在检测到p-不含分子氢的二甲苯溶剂。形成了较高分子量的胺副产物,而在底物的情况下,存在可忽略量的未反应但双键迁移的物质。除三乙胺外,还有可能使用其他烷基胺。因此,研究了使用三丁基-,三戊基-,三己基胺和N,N-二异丙基乙胺以及环状的1-乙基吡咯烷和1-乙基哌啶。作为H源的环胺和二异丙基衍生物产生的转化率最高,而具有较长烷基链的胺则显示出较小的活性。作DOI:10.1039/c7ob02614e
-
作为产物:描述:5,6-dichlorodecane 以 N,N-二甲基甲酰胺 为溶剂, 以100%的产率得到反-5-癸烯参考文献:名称:Zavada, Jiri; Krupicka, Josef; Kocian, Oldrich, Collection of Czechoslovak Chemical Communications, 1983, vol. 48, # 12, p. 3552 - 3558摘要:DOI:
-
作为试剂:参考文献:名称:Isolation of Pure Disubstituted E Olefins through Mo-Catalyzed Z-Selective Ethenolysis of Stereoisomeric Mixtures摘要:Monoaryloxide-pyrrolide (MAP) complexes of molybdenum were employed for the selective ethenolysis of 1,2-disubstituted Z olefins in the presence of the corresponding E olefins. Reactions were performed in the presence of 0.02-3.0 mol % catalyst at 22 degrees C under 20 atm ethylene. We have demonstrated that the Z isomer of an easily accessible E:Z mixture can be destroyed through ethenolysis and the E alkene thereby isolated readily in high yield and exceptional stereoisomeric purity.DOI:10.1021/ja205002v
文献信息
-
醇供氢钯催化炔烃半还原选择性合成顺、反式烯烃的方法申请人:南通大学公开号:CN109776253B公开(公告)日:2022-07-15本发明提供了一种醇供氢钯催化炔烃半还原选择性合成顺、反式烯烃的方法,包括如下步骤:将TEOA、NaOAc、催化剂、醇与炔烃于有机溶剂中进行炔烃的还原反应,反应生成顺式烯烃;将配体、催化剂、醇与炔烃于有机溶剂中进行炔烃的还原反应,反应生成反式烯烃;还原反应的反应器为密封的耐压反应器,还原反应的温度为120~150℃,还原反应的时间为20~48h;催化剂的用量为炔烃摩尔用量的5~20%,醇的用量为炔烃摩尔用量的10~100倍;R,R‑DIPAMP的用量为炔烃摩尔用量的0.5~5倍。本发明中,催化剂体系具有极高的化学反应和立体选择性,能高产率合成顺式或者反式烯烃产物;催化体系对底物的普适性强,含各种官能团的炔烃都能高效地进行高选择性地还原反应。
-
Metal-Free Epoxidation of Alkenes with Molecular Oxygen and Benzaldehyde under Visible Light Irradiation作者:Akichika Itoh、Norihiro Tada、Hiroaki Okubo、Tsuyoshi MiuraDOI:10.1055/s-0029-1218271日期:2009.11A new convenient metal-free oxidation protocol of a wide variety of alkenes with molecular oxygen and benzaldehyde under visible light irradiation of fluorescent lamp afforded their corresponding epoxides in 49―99% yields.
-
Iodine(III)-Promoted Intermolecular Diamination of Alkenes作者:José A. Souto、Yolanda González、Alvaro Iglesias、Debora Zian、Anton Lishchynskyi、Kilian MuñizDOI:10.1002/asia.201101025日期:2012.5A rapid and productive vicinal diamination of alkenes takes place in the presence of a hypervalent iodine(III) reagent and bissulfonimides as nitrogen sources. A total of more than 60 examples are presented. The reaction is characterized by its robustness and its wide substrate scope: it proceeds selectively with both terminal and internal alkenes and tolerates a range of functional groups.
-
Site-Selective Mono-Oxidation of 1,2-Bis(boronates)作者:Lu Yan、James P. MorkenDOI:10.1021/acs.orglett.9b01204日期:2019.5.17Site-selective oxidation of vicinal bis(boronates) is accomplished through the use of trimethylamine N-oxide in 1-butanol solvent. The reaction occurs with good efficiency and selectivity across a range of substrates, providing 2-hydro-1-boronic esters which are shown to be versatile intermediates in the synthesis of chiral building blocks.
-
Active metals from potassium–graphite. Palladium–graphite as catalyst in the hydrogenation of nitro-compounds, alkenes, and alkynes作者:Diego Savoia、Claudio Trombini、Achille Umani-Ronchi、Giancarlo VerardoDOI:10.1039/c39810000540日期:——Palladium–graphite, prepared by reduction of PdCl2 by means of C8K in 1,2-dimethoxyethane, is an effective catalyst for the hydrogenation of aromatic nitro-compounds, alkenes, and alkynes to anilines, alkanes, and (Z)-alkenes, respectively.
表征谱图
-
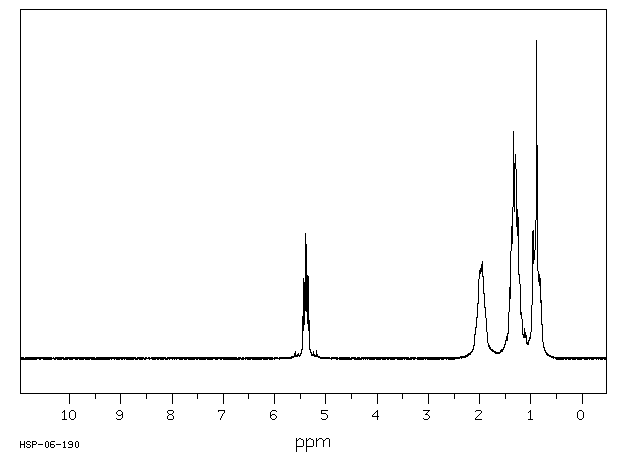
氢谱1HNMR
-
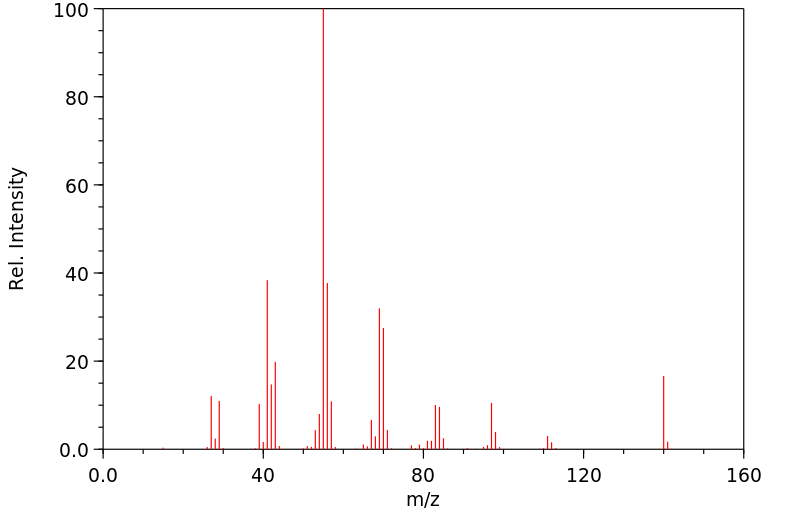
质谱MS
-
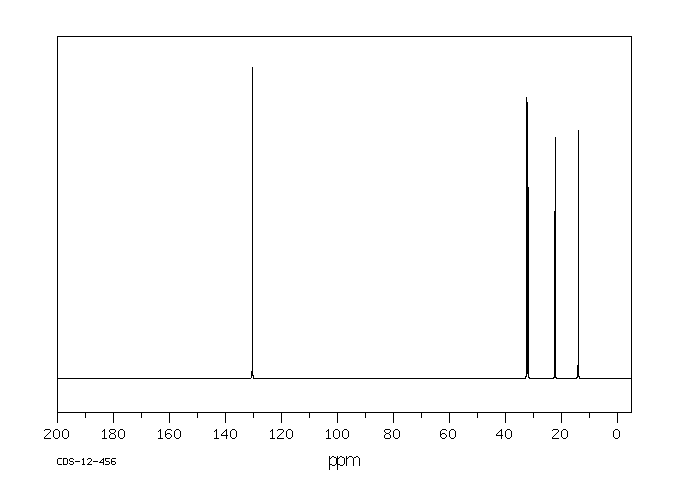
碳谱13CNMR
-
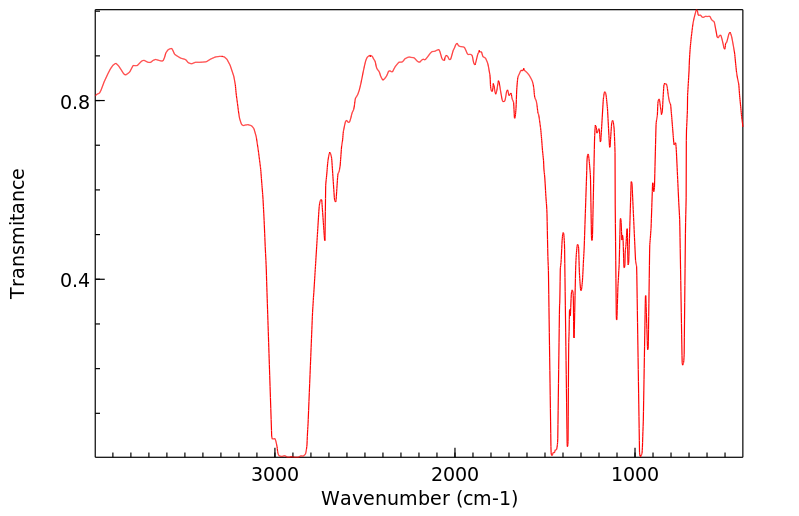
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-