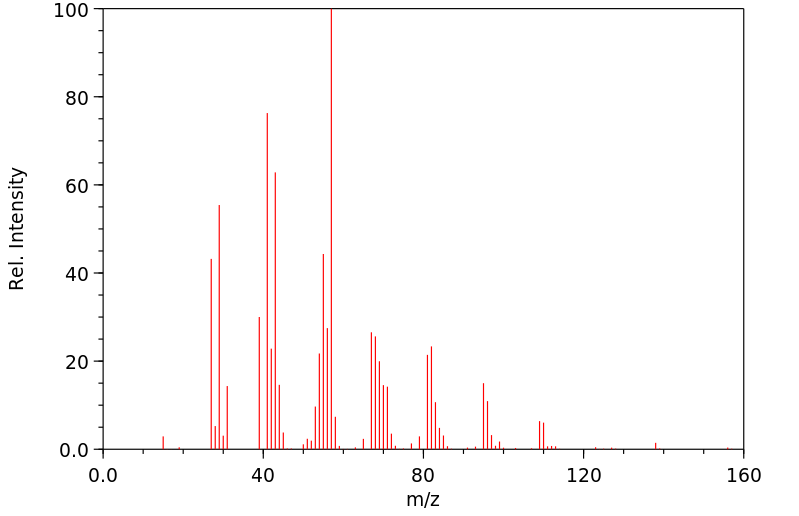
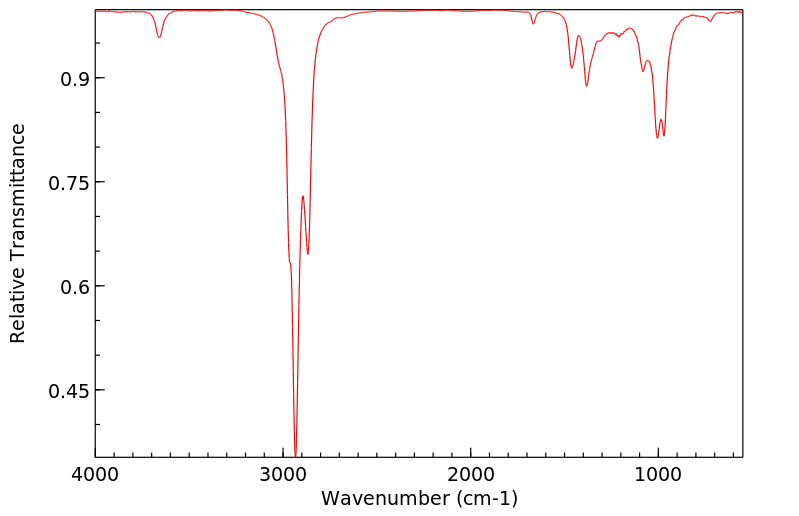
反式-2-癸烯醇 | 18409-18-2
中文名称
反式-2-癸烯醇
中文别名
2-癸烯-1-醇;反-2-癸烯-1-醇;反-2-癸烯醇
英文名称
(E)-2-decenol
英文别名
(E)-2-decen-1-ol;(E)-dec-2-en-1-ol;trans-2-decen-1-ol;2-decen-1-ol;(2E)-decen-1-ol;trans-2-decen-ol
CAS
18409-18-2
化学式
C10H20O
mdl
——
分子量
156.268
InChiKey
QOPYYRPCXHTOQZ-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:231-232 °C(lit.)
-
密度:0.844 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
LogP:3.88
-
保留指数:1254;1251;1257;1251;1254
-
稳定性/保质期:
常温常压下稳定,避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:11
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
储存条件:常温下应密闭避光保存,并保持通风和干燥。
SDS
模块 1. 化学品
1.1 产品标识符
: 反式-2-癸烯醇
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C10H20O
分子式
: 156.27 g/mol
分子量
组分 浓度或浓度范围
(E)-2-DECenol
<=100%
化学文摘登记号(CAS 18409-18-2
No.) 242-289-8
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 人员疏散到安全区域。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
用惰性吸附材料吸收并当作危险废物处理。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
231 - 232 °C - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
5.4 - (空气= 1.0)
m) 密度/相对密度
0.844 g/cm3 在 25 °C
n) 水溶性
不溶
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (2E,7Z)-deca-2,7-dien-1-ol 103983-44-4 C10H18O 154.252 —— (E)-1-(methoxymethoxy)dec-2-ene 1219000-89-1 C12H24O2 200.321 —— (E)-2-decenyl chloride 84988-53-4 C10H19Cl 174.714 反式-2-癸烯醛 (E)-2-decenal 3913-81-3 C10H18O 154.252 反式-1-溴-2-癸烯 (E)-1-bromodec-2-ene 60697-67-8 C10H19Br 219.165 甲基(E)-2-癸烯酸酯 methyl (E)-dec-2-enoate 7367-85-3 C11H20O2 184.279 —— (E)-undec-3-en-2-one 68676-86-8 C11H20O 168.279 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-4-dodecen-1-ol 81745-38-2 C12H24O 184.322 癸烯 trans-2-decene 20063-97-2 C10H20 140.269 (3E)-3-十一碳烯 trans-3-undecene 1002-68-2 C11H22 154.296 —— trans-6-tetradecene 41446-64-4 C14H28 196.376 —— (E)-1-chloromethoxy-2-decene —— C11H21ClO 204.74 壬烯二酸 trans-2-decenoic acid 334-49-6 C10H18O2 170.252 —— (E)-1-(methoxymethoxy)dec-2-ene 1219000-89-1 C12H24O2 200.321 —— (E)-2-decenyl chloride 84988-53-4 C10H19Cl 174.714 —— 1,3-decadiene 2051-25-4 C10H18 138.253 反式-2-癸烯醛 (E)-2-decenal 3913-81-3 C10H18O 154.252 反式-1-溴-2-癸烯 (E)-1-bromodec-2-ene 60697-67-8 C10H19Br 219.165 反式-2-癸醛 2-decenal 3913-71-1 C10H18O 154.252 —— (E)-4-dodecenal 174155-48-7 C12H22O 182.306 顺-7-癸烯醛 (Z)-dec-7-enal 21661-97-2 C10H18O 154.252 正癸烯 1-Decene 872-05-9 C10H20 140.269 (2E)-2-癸烯-1-基乙酸酯 (E)-2-decenyl acetate 2497-23-6 C12H22O2 198.305 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:新型碱诱导的不饱和醇异构化为醛摘要:通过使用乙二胺中的N-硫代乙二胺(LEDA-EDA)和3-氨基丙胺中的3-氨基丙酰胺锂(LAPA-APA)处理,许多烯丙基和其他不饱和醇已转化为醛。DOI:10.1039/c39850000812
-
作为产物:参考文献:名称:Delaby, Bulletin de la Societe Chimique de France, 1936, vol. <5>3, p. 2380摘要:DOI:
-
作为试剂:参考文献:名称:AGENT HAVING NEUROTROPHIC FACTOR-LIKE ACTIVITY摘要:本发明提供了一种具有高安全性和神经营养因子样活性的药物制剂,其包含任何一种含有8个碳原子(C8)或10个碳原子(C10)至12个碳原子(C12)的脂肪酸化合物或其酯类作为活性成分,例如3,7-二甲氧基辛酸乙酯、香叶醛酸乙酯等,每种都含有8个碳原子(C8),癸酸甲酯、反式-2-癸烯酸、反式-2-癸烯酸甲酯、反式-2-癸烯酸乙酯、反式-2-癸烯酸-2-癸烯酯、反式-2-癸烯酸环己酯、反式-2-癸烯酸异丙酯、反式-3-癸烯酸甲酯、反式-9-癸烯酸甲酯等,每种都含有10个碳原子(C10),反式-10-十一烯酸甲酯、反式-10-十一烯酸乙酯等,每种都含有11个碳原子(C11),以及月桂酸等,每种都含有12个碳原子(C12),或其盐或前药。公开号:US20120264959A1
文献信息
-
Convergent Synthesis of Calcium-Dependent Antibiotic CDA3a and Analogues with Improved Antibacterial Activity via Late-Stage Serine Ligation作者:Delin Chen、Kathy Hiu Laam Po、Pilar Blasco、Sheng Chen、Xuechen LiDOI:10.1021/acs.orglett.0c01544日期:2020.6.19A convergent synthesis via the late-stage serine ligation of naturally occurring calcium-dependent antibiotic CDA3a and its analogues has been developed, which allowed us to readily synthesize the analogues with the variation on the lipid tail. Some analogues were found to show 100–500-fold higher antimicrobial activity than the natural compound CDA3a against drug resistant bacteria. This study will
-
ANTI-BACTERIAL CALCIUM-DEPENDENT ANTIBIOTIC (CDA) ANALOGS AND METHODS OF TREATING BACTERIAL INFECTIONS申请人:THE UNIVERSITY OF HONG KONG公开号:US20210324009A1公开(公告)日:2021-10-21Provided herein are calcium-dependent antibiotics (CDAs), as a novel therapeutic target for treating bacterial infections. The present invention also relates to pharmaceutical compositions comprising such compounds, and to methods of use thereof for combating bacteria and treating bacterial infections.
-
Ketenimines from Isocyanides and Allyl Carbonates: Palladium-Catalyzed Synthesis of β,γ-Unsaturated Amides and Tetrazoles作者:Guanyinsheng Qiu、Mathias Mamboury、Qian Wang、Jieping ZhuDOI:10.1002/anie.201609034日期:2016.12.5allyl ethyl carbonates with isocyanides in the presence of a catalytic amount of Pd(OAc)2 provided ketenimines through β‐hydride elimination of the allyl imidoylpalladium intermediates. The insertion of the isocyanide into the π‐allyl Pd complex proceeded via an unusual η1‐allyl Pd species. The resulting ketenimines were hydrolyzed to β,γ‐unsaturated carboxamides during purification by flash column chromatography
-
Total Synthesis of the Nonenolide Xyolide Using a Regioselective Nucleophilic Epoxide Opening Approach作者:A. Narsaiah、Sachin Wadavrao、Ramesh GhogareDOI:10.1055/s-0034-1380780日期:——Abstract The total synthesis of the nonenolide xyolide is described as a convergent synthesis in 16 steps from the commercially available starting material butane-1,4-diol. The key reactions involved are: Sharpless asymmetric epoxidation, Pinnick oxidation, acid-mediated nucleophilic regioselective epoxide ring opening, Steglich esterification, and ring-closing metathesis. The total synthesis of the nonenolide
-
Oxidative Transformation of Arylmethyl Bromides and Alcohols with a Combination of Mesoporous Silica FSM-16 and Alkali Iodides under Photoirradiation作者:Akichika Itoh、Tomohiro Kodama、Shinji Inagaki、Yukio MasakiDOI:10.1021/ol016210d日期:2001.8.1see text]. A mesoporous silica FSM-16 was found to be a selective and recyclable promoter for the oxidative dehalogenation of arylmethyl bromides to provide the corresponding alcohols and for the oxidation of arylmethyl alcohols to provide the corresponding aldehydes with a combination of alkali iodides and solvents under photoirradiation conditions.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯