1,1,1,2,2-五氟-3-碘丙烷 | 354-69-8
中文名称
1,1,1,2,2-五氟-3-碘丙烷
中文别名
1-碘-2,2,3,3,3-五氟丙烷;1,1,1,2,2-五氟-3-碘代丙烷;1-碘-2,2,3,3,3-五氟丙烷,
英文名称
2,2,3,3,3-pentafluoropropyl iodide
英文别名
1,1,1,2,2-pentafluoro-3-iodopropane;2,2,3,3,3-pentafluoro-n-propyl iodide;1-iodo-2,2,3,3,3-pentafluoropropane;1H,1H-pentafluoropropyl iodide;1,1,1,2,2-Pentafluor-3-jod-propan;1-Jod-2,2,3,3,3-pentafluorpropan;Heptafluor-1-iodpropan
CAS
354-69-8
化学式
C3H2F5I
mdl
MFCD00039405
分子量
259.945
InChiKey
HENALDZJQYAUBN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:70-71°C
-
密度:2.038 g/cm3(Temp: 25 °C)
-
闪点:70-71°C
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:9
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:5
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2903799090
-
储存条件:室温
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,1,1,2,2-五氟丙烷 1,1,1,2,2-pentafluoropropane 1814-88-6 C3H3F5 134.049
反应信息
-
作为反应物:描述:1,1,1,2,2-五氟-3-碘丙烷 在 三正丁基氢锡 作用下, 反应 2.0h, 以91%的产率得到1,1,1,2,2-五氟丙烷参考文献:名称:氢化三丁基锡对多卤氟烃的还原脱卤作用摘要:用三丁基锡氢化物还原ClCF 2 CFClCF 2 Cl,(ClCF 2 CFCl)2,ICH 2(CF 2)3 CH 2 I和邻位二氯全氟环烷烃在内的多卤代氟烃,可以以良好的收率很好地得到相应的氢氟烃。将结果与其他还原剂的类似还原反应以及非氟化类似物的氢化锡还原反应进行了比较。DOI:10.1016/0022-1139(94)03153-q
-
作为产物:描述:以 solid 为溶剂, 生成 1,1,1,2,2-五氟-3-碘丙烷参考文献:名称:1-Iodo-polyfluoroalkanes from polyfluoroalkoxy trimethylsilanes and iodochloro triphenylphosphorane摘要:Polyfluoroalkoxy trimethylsilanes R(f)CH(2)OSi(CH3)(3) (from the alcohols R(f)CH(2)OH and HMDS), react with Ph(3)PICl (from ICl and Ph(3)P) eliminating (CH3)(3)SiCl. Pyrolysis or the residues gives Ph(3)PO and pure iodides R(f)CH(2).DOI:10.1016/s0040-4039(00)73201-1
文献信息
-
<i>Z</i> ‐Selective Fluoroalkenylation of (Hetero)Aromatic Systems by Iodonium Reagents in Palladium‐Catalyzed Directed C−H Activation作者:Balázs L. Tóth、Gergő Sályi、Attila Domján、Orsolya Egyed、Attila Bényei、Zsombor Gonda、Zoltán NovákDOI:10.1002/adsc.202101108日期:2022.1.18The direct and catalytic incorporation of fluorine containing molecular motifs into organic compounds resulting high-value added chemicals represents a rapidly evolving part of synthetic methodologies, thus this area is in the focus of pharmaceutical and agrochemical research. Herein we report a stereoselective procedure for direct fluorovinylation of aromatic and heteroaromatic scaffolds. This methodology
-
An Olefinic 1,2‐Boryl‐Migration Enabled by Radical Addition: Construction of <i>gem</i> ‐Bis(boryl)alkanes作者:Binlin Zhao、Zexian Li、Yixiao Wu、Yandong Wang、Jiasheng Qian、Yu Yuan、Zhuangzhi ShiDOI:10.1002/anie.201903721日期:2019.7.8(commercially available or prepared from alkenyl bromides and Mg) with B2Pin2 (bis(pinacolato)diboron) react with diverse alkyl halides by a Ru photocatalyst to give various gem‐bis(boryl)alkanes. Alkyl radicals add efficiently to the alkenyl diboronate complexes, and the adduct radical anions undergo radical‐polar crossover, specifically, a 1,2‐boryl‐anion shift from boron to the α‐carbon sp2 center.
-
Photocatalytic Palladium-Catalyzed Fluoroalkylation of Styrene Derivatives作者:Réka Adamik、Tamás Földesi、Zoltán NovákDOI:10.1021/acs.orglett.0c03043日期:2020.10.16A visible light induced palladium-catalyzed fluoroalkylation method was developed. The Heck-type alkyl coupling reaction enables the introduction of trifluoroethyl, difluoroethyl and other fluoroalkyl fragment into styrenes under mild reaction conditions without the use of additional photosensitizers and ensures access to fluoroalkylated olefins on a broad scale.
-
Terminally perfluorinated long-chain alkanethiols作者:Michael Graupe、Thomas Koini、Vincent Y Wang、George M Nassif、Ramon Colorado、Ramon J Villazana、Henry Dong、Yasuhiro F Miura、Olga E Shmakova、T.Randall LeeDOI:10.1016/s0022-1139(98)00284-x日期:1999.2good yields under free radical conditions. Reduction of the resulting secondary iodides gave long-chain alkanethioacetates with perfluoroalkyl terminal segments. These intermediates were readily transformed into the corresponding terminally perfluorinated alkanethiols by acidic deprotection. The product thiols should find use in the generation of well-defined fluorinated interfaces using the self-assembled
-
Photocatalytic Reductive Fluoroalkylation of Nitrones作者:Vyacheslav I. Supranovich、Vitalij V. Levin、Marina I. Struchkova、Alexander D. DilmanDOI:10.1021/acs.orglett.7b03987日期:2018.2.2A method for the addition of fluorinated groups to nitrones using an iridium photocatalyst and ascorbic acid as a stoichiometric reducing agent is described. The reaction proceeds through the generation of fluorinated radicals by single-electron reduction of fluorinated alkyl iodides with an iridium complex mediated by visible light. Besides perfluorinated reagents, partially fluorinated alkyl iodides
表征谱图
-
氢谱1HNMR
-
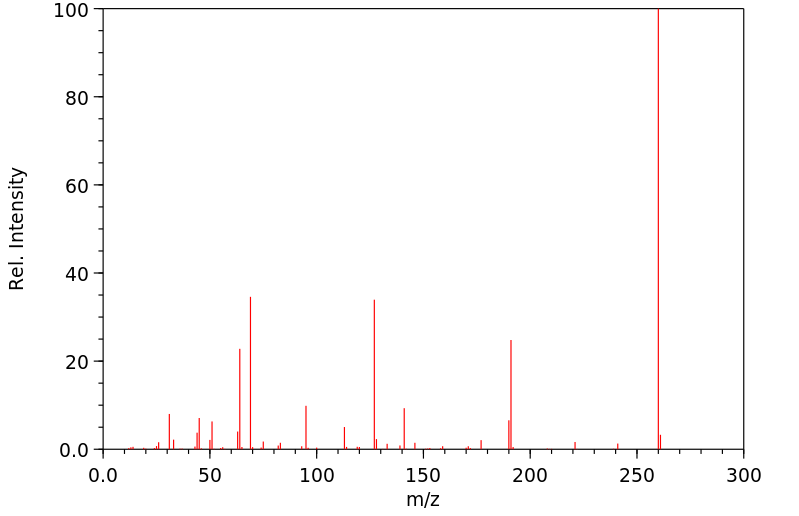
质谱MS
-
碳谱13CNMR
-
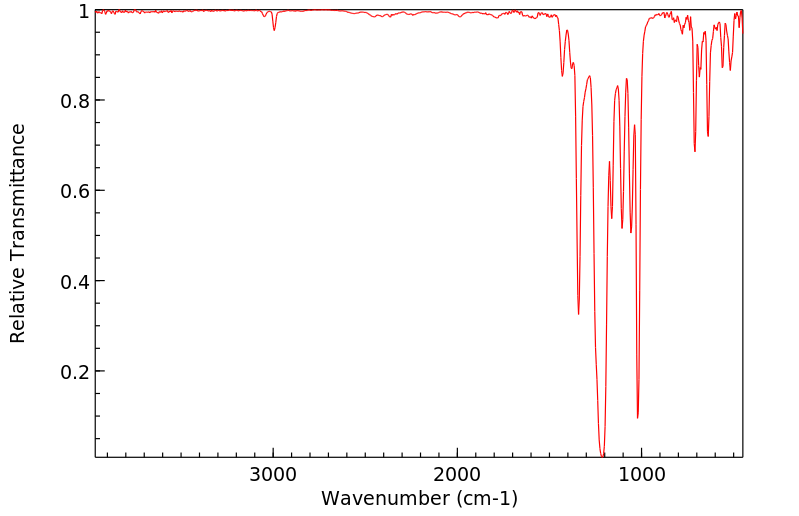
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
胍,N-[3-(氨基甲基)-5-甲基苯基]-N'-乙基-
碘甲烷
碘甲基环辛烷
碘甲基环戊烷
碘环庚烷
碘环十二烷
碘环丁烷
碘十六烷
碘代环戊烷
碘代正辛烷-D2
碘代异丁烷
碘代叔丁烷
碘代丙烷-D7
碘代丙烷-D3
碘代丙烷-D2
碘代丙烷-D2
碘乙烷-d<
碘乙烷-D1
碘乙烷-2-13C
碘乙烷-2,2,2-d3
碘乙烷-1-13C
碘乙烷-1,1-d2
碘乙烷(1,2-13C2)
碘乙烷
碘丁烷-D9
碘(碘甲氧基)甲烷
甲基碘化钙
环辛烷,1-氟-2-碘-,反-
环戊二烯并[1,3]环丙烯并[1,2]环庚烯-2(1H)-酮,八氢-3a,5,5-三甲基-,(3aR,3bR,8aS)-rel-
环丙基碘
无花果蛋白酶来源于无花果树乳胶
新戊氧基
新戊基碘
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
异戊基碘
异丁基锰(II)碘化物
反式-4-己烯基碘
十氢-2-(碘甲基)-萘
十四烷基碘化物
十五氟碘庚烷
十九氟-9-碘壬烷
全氟辛基碘烷
全氟碘代丁烷
全氟异戊基碘
全氟异庚基碘化物
全氟异壬基碘
全氟异十一烷基碘化物
全氟己基碘烷
全氟叔丁基碘化物