2,4-二甲基喹啉 | 1198-37-4
中文名称
2,4-二甲基喹啉
中文别名
2,4-二甲基-1-氮杂萘;4-甲基喹哪啶;2,4-二甲基(5,6)苯并吡啶;2,4-二甲喹啉
英文名称
2,4-dimethylquinoline
英文别名
——
CAS
1198-37-4
化学式
C11H11N
mdl
MFCD00006760
分子量
157.215
InChiKey
ZTNANFDSJRRZRJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:14.85°C
-
沸点:264-265 °C(lit.)
-
密度:1.061 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
溶解度:可溶于氯仿(少量)、DMSO(少量)、甲醇(少量)
-
蒸汽压力:0.01 mmHg
-
保留指数:1417;1434;1417;1434;1418;1446;1497;1446
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,不存在已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.181
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38,R36/38
-
WGK Germany:3
-
海关编码:29334900
-
危险性防范说明:P305+P351+P338
-
危险性描述:H315,H319
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境具有良好的通风或排气设施。
SDS
| Name: | 2 4-Dimethylquinoline 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1198-37-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1198-37-4 | 2,4-Dimethylquinoline | 97% | 214-832-9 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1198-37-4: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 264 - 265 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 113 deg C ( 235.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.061
Molecular Formula: C11H11N
Molecular Weight: 157.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1198-37-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,4-Dimethylquinoline - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 1198-37-4: No information available.
Canada
CAS# 1198-37-4 is listed on Canada's DSL List.
CAS# 1198-37-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1198-37-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
喹啉类化合物凭借其重要的生物和药理活性,在制药、农药及染料等领域获得了广泛应用。特别是在药物合成领域,作为一类极其重要的含氮杂环化合物,喹啉类化合物因具有抗菌、抗病毒、抗癌、抗肿瘤等多种多样的生物和药理活性,成为新药物设计与研发的热点之一。
制备近几年,由于其操作流程简单、反应时间短及高产率等优点,化学催化法已成为合成喹啉类化合物的主要方法。用于催化合成喹啉类化合物的路径通常包括Skraup合成法、Combes合成法、Doebner-Von Miller合成法、Knorr合成法、Friedlander法、Conrad-Limpach合成法以及Pfitzinger合成法等。在这些化学合成路径中,常常需要多种催化剂参与,例如强酸、强碱、无机盐和重金属等。
2,4-二甲基喹啉的合成反应式如下图所示:
化学性质无色到淡黄色液体,沸点为264-265℃(143℃/2.0kPa),相对密度为1.0611(15/4℃),折光率为1.6075。易溶于醇和醚,极微溶于水。
用途主要用于染料中间体的合成。
生产方法将苯胺与碘一起加热至170-175℃,然后滴加丙酮并强烈搅拌,同时收集馏出物。从反应混合物中减压蒸馏分离出苯胺和中间馏分2,2,4-三甲基-1,2-二氢喹啉。后者与无水苯胺、金属钠及铜粉一起加热反应,经过减压分馏后可得到2,4-二甲基喹啉,收率可达80-90%。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-甲基喹啉-2-甲醛 4-methylquinoline-2-carbaldehyde 40105-30-4 C11H9NO 171.199 —— (4-methyl-quinolin-2-yl)-methanol 33787-85-8 C11H11NO 173.214 (2-甲基-4-喹啉)-甲醇 (2-methylquinolin-4-yl)methanol 4939-28-0 C11H11NO 173.214 7-溴-2,4-二甲基-喹啉 7-bromo-2,4-dimethyl-quinoline 103858-50-0 C11H10BrN 236.111 4-甲基喹啉 4-methylquinoline 491-35-0 C10H9N 143.188 2,4-二甲基喹啉1-氧化物 2,4-dimethylquinoline 1-oxide 14300-12-0 C11H11NO 173.214 2-甲基喹啉 2-methylquinoline 91-63-4 C10H9N 143.188 4-甲基喹啉-2-羧酸 4-methyl-quinoline-2-carboxylic acid 40609-76-5 C11H9NO2 187.198 勒皮啶 N-氧化物 4-methylquinoline 1-oxide 4053-40-1 C10H9NO 159.188 4-甲基-2-氯-喹啉 2-chloro-4-methylquinoline 634-47-9 C10H8ClN 177.633 —— 2-d-4-methylquinoline 20668-24-0 C10H9N 144.18 4-甲基喹啉-2-羧酸乙酯 ethyl 4-methylquinoline-2-carboxylate 142729-99-5 C13H13NO2 215.252 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-ethyl-4-methylquinoline 33357-44-7 C12H13N 171.242 4-甲基喹啉-2-甲醛 4-methylquinoline-2-carbaldehyde 40105-30-4 C11H9NO 171.199 —— (4-methyl-quinolin-2-yl)-methanol 33787-85-8 C11H11NO 173.214 —— 2-(2-Hydroxyethyl)-4-methylquinoline 55908-37-7 C12H13NO 187.241 —— quinoline-2,4-dicarboxaldehyde 52287-46-4 C11H7NO2 185.182 2,4-二甲基喹啉1-氧化物 2,4-dimethylquinoline 1-oxide 14300-12-0 C11H11NO 173.214 —— 4-methyl-2(E)-styrylquinoline 84586-45-8 C18H15N 245.324 2,4-二甲基喹啉-8-胺 8-amino-2,4-dimethylquinoline 3393-70-2 C11H12N2 172.23 8-氯-2,4-二甲基喹啉 8-chloro-2,4-dimethylquinoline 67358-87-6 C11H10ClN 191.66 4-甲基喹啉-2-羧酸 4-methyl-quinoline-2-carboxylic acid 40609-76-5 C11H9NO2 187.198 —— 2,4-dimethyl-quinolin-3-ol 103988-88-1 C11H11NO 173.214 2-甲基喹啉-4-甲酸 2-methylquinoline-4-carboxylic acid 634-38-8 C11H9NO2 187.198 —— 2,4-dimethyl-6-nitro-quinoline 84264-42-6 C11H10N2O2 202.213 4-甲基喹啉-2-羧酸甲酯 methyl 4-methylquinoline-2-carboxylate 80109-83-7 C12H11NO2 201.225 —— 2,4-di-trans-styryl-quinoline 103327-32-8 C25H19N 333.433 2-甲基-喹啉-4-羧酸甲酯 methyl 2-methylquinoline-4-carboxylate 55625-40-6 C12H11NO2 201.225 —— 2-methyl-4-phenacylquinoline 65845-65-0 C18H15NO 261.323 —— 2,4-dimethyl-8-nitroquinoline 2801-28-7 C11H10N2O2 202.213 —— 2,4-dimethyl-5-nitro-quinoline 351354-98-8 C11H10N2O2 202.213 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Bamberger; Brady, Chemische Berichte, 1900, vol. 33, p. 3652摘要:DOI:
-
作为产物:描述:参考文献:名称:Ultrasound-promoted coupling of aryl and heteroaryl halides in the presence of lithium wire摘要:Although sonochemical reactions of bromobenzene and of 2-, 3- and 4-halogenopyridines with lithium wire in THF solution yield the expected dimers, considerable dehalogenation also occurs, as monitored by C-13-NMR spectroscopy. A pathway for the unusual formation of 4,4'-bipyridyl from the 2-halogenopyridines is proposed. No dimers were detected from the reactions with 2-bromo-4-methylquinoline and 7-bromo-2,4-dimethylquinoline. The synthetic potential of their alternative facile sonochemical dehalogenation is propounded.DOI:10.1007/bf00809805
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
ANTHELMINTIC COMPOUNDS AND COMPOSITIONS AND METHOD OF USING THEREOF申请人:Meng Charles Q.公开号:US20140142114A1公开(公告)日:2014-05-22The present invention relates to novel anthelmintic compounds of formula (I) below: wherein Y and Z are independently a bicyclic carbocyclic or a bicyclic heterocyclic group, or one of Y or Z is a bicyclic carbocyclic or a bicyclic heterocyclic group and the other of Y or Z is alkyl, alkenyl, alkynyl, cycloalkyl, phenyl, heterocyclyl or heteroaryl, and variables X 1 , X 2 , X 3 , X 4 , X 5 , X 6 , X 7 and X 8 are as defined herein. The invention also provides for veterinary compositions comprising the anthelmintic compounds of the invention, and their uses for the treatment and prevention of parasitic infections in animals.本发明涉及以下式(I)的新型驱虫化合物: 其中 Y和Z分别是双环碳环或双环杂环基团,或者Y或Z中的一个是双环碳环或双环杂环基团,另一个是烷基,烯基,炔基,环烷基,苯基,杂环基或杂芳基,以及变量X 1 ,X 2 ,X 3 ,X 4 ,X 5 ,X 6 ,X 7 和X 8 如本文所定义。本发明还提供了包含本发明的驱虫化合物的兽药组合物,以及它们用于治疗和预防动物寄生虫感染的用途。
-
Organocatalyzed, Visible-Light Photoredox-Mediated, One-Pot Minisci Reaction Using Carboxylic Acids via <i>N</i>-(Acyloxy)phthalimides作者:Trevor C. Sherwood、Ning Li、Aliza N. Yazdani、T. G. Murali DharDOI:10.1021/acs.joc.8b00205日期:2018.3.2one-pot Minisci reaction has been developed using visible light, an organic photocatalyst, and carboxylic acids as radical precursors via the intermediacy of in situ-generated N-(acyloxy)phthalimides. The conditions employed are mild, demonstrate a high degree of functional group tolerance, and do not require a large excess of the carboxylic acid reactant. As a result, this reaction can be applied to
-
[EN] MONOACYLGLYCEROL LIPASE MODULATORS<br/>[FR] MODULATEURS DE LA MONOACYLGLYCÉROL LIPASE申请人:JANSSEN PHARMACEUTICA NV公开号:WO2021191359A1公开(公告)日:2021-09-30Fused and bridged compounds of Formula (I), and pharmaceutically acceptable salts, isotopes, N-oxides, solvates, and stereoisomers thereof, pharmaceutical compositions containing them, methods of making them, and methods of using them including methods for treating disease states, disorders, and conditions associated with MGL modulation, such as those associated with pain, psychiatric disorders, neurological disorders (including, but not limited to major depressive disorder, treatment resistant depression, anxious depression, autism spectrum disorders, Asperger syndrome, bipolar disorder), cancers and eye conditions: (I) wherein R1a, R1b, R2, and R3, are defined herein.化合物的融合和桥联化合物的化学式(I),以及其药用可接受的盐、同位素、N-氧化物、溶剂合物和立体异构体,含有它们的药物组合物,制备它们的方法,以及使用它们的方法,包括用于治疗与MGL调节相关的疾病状态、紊乱和症状的方法,例如与疼痛、精神紊乱、神经紊乱(包括但不限于重度抑郁症、治疗抵抗性抑郁症、焦虑性抑郁症、自闭症谱系障碍、亚斯伯格综合症、双相情感障碍)、癌症和眼部疾病相关的方法:(I)其中R1a、R1b、R2和R3在此定义。
表征谱图
-
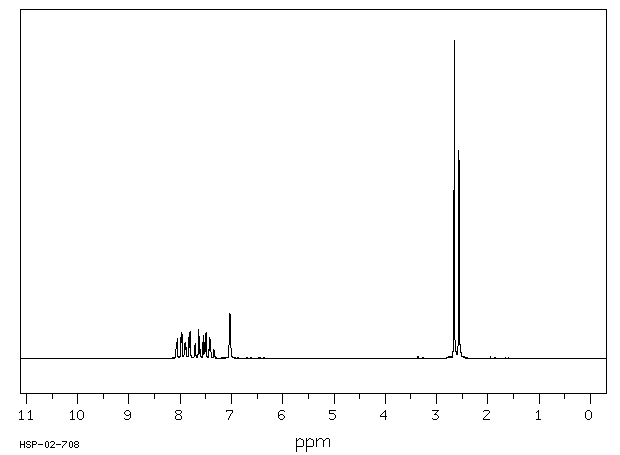
氢谱1HNMR
-
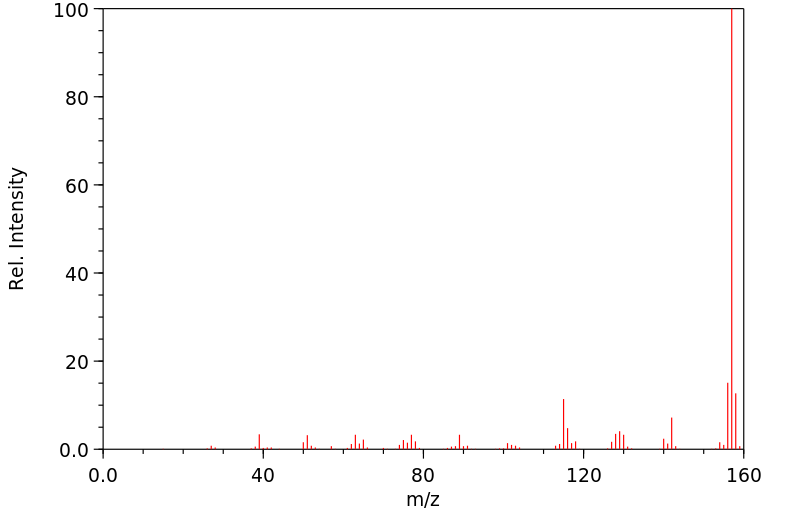
质谱MS
-
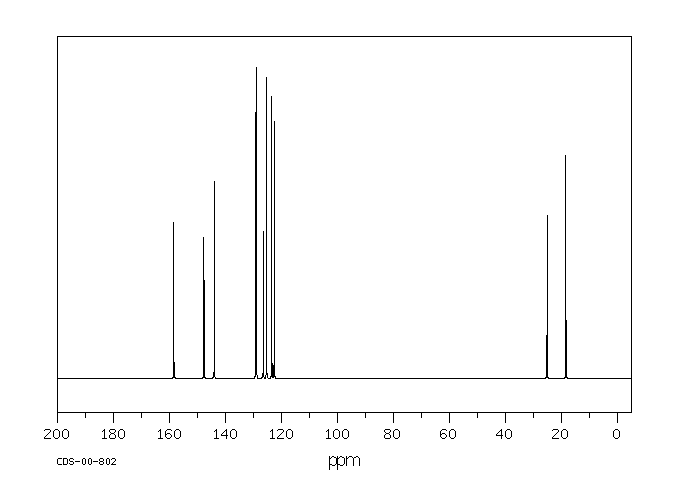
碳谱13CNMR
-
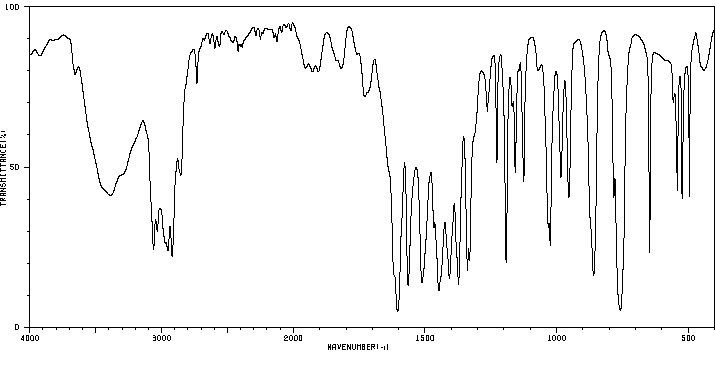
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43