4-氨基-3-硝基苯甲酸 | 1588-83-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:280 °C (dec.) (lit.)
-
沸点:315.51°C (rough estimate)
-
密度:1.5181 (rough estimate)
-
闪点:290°C
-
溶解度:>27.3 [ug/mL]
-
稳定性/保质期:
在常温常压下稳定,应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:109
-
氢给体数:2
-
氢受体数:5
安全信息
-
TSCA:Yes
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2922499990
-
危险品运输编号:NONH for all modes of transport
-
危险类别:IRRITANT
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
: 4-Amino-3-nitrobenzoic acid
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如果在皮肤上: 用大量肥皂和水淋洗。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/ 就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C7H6N2O4
分子式
: 182.13 g/mol
分子量
成分 浓度
4-Amino-3-nitrobenzoic acid
-
化学文摘编号(CAS No.) 1588-83-6
EC-编号 216-453-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用大量水彻底冲洗至少15分钟并请教医生。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 280 °C - 分解
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
用途:用作染料及医药中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二硝基苯甲酸 3,4-dinitrobenzoic acid 528-45-0 C7H4N2O6 212.119 —— 4-formylamino-3-nitro-benzoic acid 26113-31-5 C8H6N2O5 210.146 间硝基苯甲酸 3-nitrobenzoic acid 121-92-6 C7H5NO4 167.121 4-乙酰胺基-3-硝基苯甲酸 3-nitro-4-acetamidobenzoic acid 1539-06-6 C9H8N2O5 224.173 3,4-二硝基甲苯 3,4-Dinitrotoluene 610-39-9 C7H6N2O4 182.136 4-氯-3-硝基苯甲酸 4-chloro-3-nitrobenzoate 96-99-1 C7H4ClNO4 201.566 4-氟-3-硝基苯甲酸 3-nitro-4-fluorobenzoic acid 453-71-4 C7H4FNO4 185.111 4-氨基-3-硝基苯甲腈 4-Amino 3-nitro-benzonitrile 6393-40-4 C7H5N3O2 163.136 4-甲氧基-3-硝基苯甲酸 4-methoxy-3-nitrobenzoic acid 89-41-8 C8H7NO5 197.147 2-硝基-4-(2-氯乙酰基)-乙酰苯胺 N-(4-(2-chloroacetyl)-2-nitrophenyl)acetamide 119457-11-3 C10H9ClN2O4 256.645 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(羟基氨基)-3-硝基苯甲酸 3-nitro-4-hydroxyaminobenzoic acid 15150-93-3 C7H6N2O5 198.135 4-氨基-3-硝基苯甲酸甲酯 methyl 4-amino-3-nitrobenzoate 3987-92-6 C8H8N2O4 196.163 4-氨基-3-硝基苯甲酸乙酯 ethyl 4-amino-3-nitrobenzoate 76918-64-4 C9H10N2O4 210.189 —— iso-propyl 4-amino-3-nitrobenzoate 6083-78-9 C10H12N2O4 224.216 —— n-Propyl 4-amino-3-nitrobenzoate 121649-59-0 C10H12N2O4 224.216 4-氨基-3-硝基苯甲酸苄酯 benzyl 4-amino-3-nitrobenzoate 1004647-09-9 C14H12N2O4 272.26 —— iso-Butyl 4-amino-3-nitrobenzoate 121649-60-3 C11H14N2O4 238.243 —— 3-Nitro-4-aminobenzoic acid-n-butyl ester 41680-92-6 C11H14N2O4 238.243 —— 4-Azido-3-nitro-benzoesaeure 54974-60-6 C7H4N4O4 208.133 (4-氨基-3-硝基苯基)甲醇 (4-amino-3-nitrophenyl)methanol 63189-97-9 C7H8N2O3 168.152 —— 2-nitro-4-[heptadecoxycarbonyl]aniline —— C24H40N2O4 420.593 间硝基苯甲酸 3-nitrobenzoic acid 121-92-6 C7H5NO4 167.121 —— 4-amino-3-chloro-5-nitro-benzoic acid 37902-01-5 C7H5ClN2O4 216.581 4-氨基-3-溴-5-硝基苯甲酸 3-bromo-4-amino-5-nitrobenzoic acid 556651-33-3 C7H5BrN2O4 261.032 —— 4-amino-3-iodo-5-nitrobenzoic acid 89677-77-0 C7H5IN2O4 308.032 —— 4-Amino-3-nitrobenzoyl chloride 121649-58-9 C7H5ClN2O3 200.581 —— 4'-dimethylamino-2-nitroazobenzene-4-carboxylic acid 338445-46-8 C15H14N4O4 314.301 —— 4'-dimethylamino-2-nitroazobenzene-4-carboxylic acid 392300-99-1 C15H14N4O4 314.301 4-氨基-3-硝基苯甲酰胺 4-amino-3-nitrobenzamide 41263-65-4 C7H7N3O3 181.151 4-氨基-3-氯-5-硝基苯甲酸甲酯 methyl 4-amino-5-chloro-3-nitrobenzoate 863886-04-8 C8H7ClN2O4 230.608 4-乙酰氨基-3-硝基苯甲酸甲酯 methyl 4-acetamido-3-nitrobenzoate 6313-39-9 C10H10N2O5 238.2 —— methyl 4-(benzylamino)-3-nitrobenzoate 68502-46-5 C15H14N2O4 286.287 4-氨基-3-溴-5-硝基苯甲酸甲酯 methyl 4-amino-3-bromo-5-nitrobenzoate 105655-17-2 C8H7BrN2O4 275.059 4-氨基-3-碘-5-硝基苯甲酸甲酯 methyl 4-amino-3-iodo-5-nitrobenzoate 172221-28-2 C8H7IN2O4 322.059 4-氨基-3-乙烯基-5-硝基苯甲酸甲酯 methyl 4-amino-3-nitro-5-vinylbenzoate 918446-47-6 C10H10N2O4 222.2 4-((2-乙氧基-2-氧代乙基)氨基)-3-硝基苯甲酸甲酯 methyl 3-nitro-4-((2-ethoxy-2-oxoethyl) amino)benzoate 1381944-43-9 C12H14N2O6 282.253 —— 4-amino-3-nitrobenzohydrazide 205652-97-7 C7H8N4O3 196.166 —— 4-amino-N,N-dimethyl-3-nitrobenzamide 726206-82-2 C9H11N3O3 209.205 —— 2-nitro-4-carboxy-4'-[N,N-di(2-hydroxyethyl)amino]azobenzene —— C17H18N4O6 374.353 —— 4-{[(tert-butyldimethylsilyl)oxy]methyl}-2-nitroaniline 1448350-06-8 C13H22N2O3Si 282.415 —— methyl 4-[(tert-butoxy)carbonylamino]-3-nitrobenzoate 327046-64-0 C13H16N2O6 296.28 —— 4-amino-N-(benzyloxy)-3-nitrobenzamide —— C14H13N3O4 287.275 4-羟基-3-硝基苯甲酸 3-nitro-4-hydroxybenzoic acid 616-82-0 C7H5NO5 183.12 3,4-二氨基苯甲酸 3,4-diaminobenzoic acid 619-05-6 C7H8N2O2 152.153 4-碘-3-硝基苯甲酸 4-iodo-3-nitrobenzoic acid 35674-27-2 C7H4INO4 293.018 4-氨基-N-丁基-3-硝基苯甲酰胺 4-amino-N-butyl-3-nitrobenzamide 88638-67-9 C11H15N3O3 237.258 —— 4-ethoxalylamino-3-nitrobenzoic acid 14121-59-6 C11H10N2O7 282.21 4-碘-3-硝基苯甲酸甲酯 4-iodo-3-nitrobenzoic acid methyl ester 89976-27-2 C8H6INO4 307.044 4-氰基-3-硝基苯甲酸 4-cyano-3-nitrobenzoic acid 153775-42-9 C8H4N2O4 192.131 4-氨基-3-硝基-N-苯基苯甲酰胺 4-amino-3-nitro-N-phenylbenzamide 89790-71-6 C13H11N3O3 257.249 —— 4-chloro-benzoic acid N'-(4-amino-3-nitro-benzoyl)-hydrazide 1137671-43-2 C14H11ClN4O4 334.719 3,4-二氨基苯甲酸甲酯 Methyl 3,4-diaminobenzoate 36692-49-6 C8H10N2O2 166.18 —— 4-[4'-(N,N-methacryloyloxy-2-ethyl-ethylamino)phenyl]-3-nitro-benzoic acid 176648-76-3 C21H22N4O6 426.429 —— 4-amino-N-cyclohexyl-3-nitrobenzamide 89790-70-5 C13H17N3O3 263.296 —— 4-amino-N′-(4-methoxybenzoyl)-3-nitrobenzohydrazide 1137671-55-6 C15H14N4O5 330.3 —— methyl 4-ethynyl-3-nitrobenzoate 1374035-38-7 C10H7NO4 205.17 —— methyl 3-bromo-4-[(E/Z)-but-2-enylamino]-5-nitrobenzoate 881909-48-4 C12H13BrN2O4 329.15 4-氰基-3-硝基苯甲酸甲酯 methyl 4-cyano-3-nitrobenzoate 1033997-01-1 C9H6N2O4 206.158 4-氨基-3-硝基苯甲酸吗啉 (4-amino-3-nitrophenyl)(morpholino)methanone 89790-86-3 C11H13N3O4 251.242 3,4-二氨基苯甲酸乙酯 ethyl 3,4-diaminobenzoate 37466-90-3 C9H12N2O2 180.206 —— 4-amino-N-(3-chloro-phenyl)-3-nitrobenzamide 1021165-56-9 C13H10ClN3O3 291.694 4-氨基-N-(3,4-二甲基苯基)-3-硝基苯甲酰胺 4-amino-N-(3,4-dimethylphenyl)-3-nitrobenzamide 1021165-64-9 C15H15N3O3 285.302 —— methyl 4-amino-3-(1-methylenepropyl)-5-nitrobenzoate 918446-48-7 C12H14N2O4 250.254 丙基3,4-二氨基苯甲酸酯 n-Propyl 3,4-diaminobenzoate 92396-76-4 C10H14N2O2 194.233 —— 4-amino-N-(4-tert-butylphenyl)-3-nitro-benzamide 1021166-02-8 C17H19N3O3 313.356 异丁基3,4-二氨基苯甲酸酯 iso-butyl 3,4-diaminobenzoate 121649-62-5 C11H16N2O2 208.26 —— butyl 3,4-diaminobenzoate 63655-47-0 C11H16N2O2 208.26 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:参考文献:名称:Salkowski, Justus Liebigs Annalen der Chemie, 1874, vol. 173, p. 50,51摘要:DOI:
-
作为产物:描述:参考文献:名称:N-氧化物和相关化合物。第二十八部分。5-氨基和5-羟基苯并呋喃摘要:描述了多种5-取代的苯并呋喃喃及其前体的合成。5-乙酰氧基-和5-酰基氨基-苯并呋喃的仔细水解导致分离出5-羟基-和5-氨基-苯并呋喃盐酸盐。讨论了这些化合物的结构。DOI:10.1039/j39660000971
-
作为试剂:描述:(2S,4S)-5-<(tert-butyldimethylsilyl)oxy>-2,4-dimethyl-pentan-1-ol 在 正丁基锂 、 N-羟基-7-氮杂苯并三氮唑 、 2,2,6,6-四甲基哌啶氧化物 、 2,4,6-三氯苯甲酸 、 三氯异氰尿酸 、 [(S)-(-)-5,5'-bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole][4-cyano-3-nitrobenzenecarboxylato][1,2,3-η-2-propenyl]iridium(III) 、 potassium tert-butylate 、 氢气 、 碳酸氢钠 、 caesium carbonate 、 三乙胺 、 N,N-二异丙基乙胺 、 4-氨基-3-硝基苯甲酸 、 N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate 、 palladium dichloride 作用下, 以 四氢呋喃 、 甲醇 、 正己烷 、 二氯甲烷 、 水 、 甲苯 为溶剂, -78.0~100.0 ℃ 、101.33 kPa 条件下, 反应 114.53h, 生成 (3S,4R,5S,7S,8R)-3,5,7-trimethyl-8-((trimethylsilyl)oxy)undec-10-en-4-yl hept-6-enoyl-L-valinate参考文献:名称:羟内酰胺A的全合成摘要:以高效且立体控制的方式实现了强效的多药耐药逆转剂dysoxylacatam A(1)的总合成。该策略的重点是锂化和硼化策略的迭代组合,包括Aggarwal同源和Matteson同源,Brown crotylation,Krische allylation和闭环易位,以构筑大环。DOI:10.1021/acs.orglett.0c00074
文献信息
-
Synthesis of Indoles: Efficient Functionalisation of the 7-Position作者:Emmanuel Demont、Nicolas Charrier、Rachel Dunsdon、Graham Maile、Alan Naylor、Alistair O’Brien、Sally Redshaw、Pam Theobald、David Vesey、Daryl WalterDOI:10.1055/s-2006-950223日期:2006.10Traditional strategies in indole chemistry do not allow high-yielding access to some substitution patterns such as 3,5,7-trisubstituted indoles. We report in this article the efficient synthesis of this type of indole. The Heck cyclisation strategy we used allows the synthesis of 7-iodo-, 7-alkoxy-, 7-amino-, and 7-nitroindoles bearing other functionalities at the 3- and 5-positions. We believe that
-
作为细胞坏死阻碍剂的吲哚化合物
-
Benzoic Acid Derivatives with Trypanocidal Activity: Enzymatic Analysis and Molecular Docking Studies toward Trans-Sialidase作者:Muhammad Kashif、Antonio Moreno-Herrera、Juan Villalobos-Rocha、Benjamín Nogueda-Torres、Jaime Pérez-Villanueva、Karen Rodríguez-Villar、José Medina-Franco、Peterson de Andrade、Ivone Carvalho、Gildardo RiveraDOI:10.3390/molecules22111863日期:——target for new anti-Chagas drugs. In this work, the aims were to design and find a new series of benzoic acid derivatives as trans-sialidase (TS) inhibitors and anti-trypanosomal agents. Three compounds (14, 18, and 19) sharing a para-aminobenzoic acid moiety showed more potent trypanocidal activity than the commercially available drugs nifurtimox and benznidazole in both strains: the lysis concentration
-
[EN] P2X3, RECEPTOR ANTAGONISTS FOR TREATMENT OF PAIN<br/>[FR] ANTAGONISTES DU RÉCEPTEUR P2X3 POUR LE TRAITEMENT DE LA DOULEUR申请人:MERCK SHARP & DOHME公开号:WO2010111058A1公开(公告)日:2010-09-30The subject invention relates to novel P2X3 receptor antagonists that play a critical role in treating disease states associated with pain, in particular peripheral pain, inflammatory pain, or tissue injury pain that can be treated using a P2X3 receptor subunit modulator.该发明涉及新型P2X3受体拮抗剂,其在治疗与疼痛相关的疾病状态中发挥关键作用,特别是可以使用P2X3受体亚单位调节剂来治疗的外周疼痛、炎症性疼痛或组织损伤疼痛。
-
Synthesis, Molecular Docking Analysis and Biological Evaluations of Saccharide-Modified Thiadiazole Sulfonamide Derivatives作者:Zuo-Peng Zhang、Ye Zhong、Zhen-Bin Han、Lin Zhou、Hua-Sheng Su、Jian Wang、Yang Liu、Mao-Sheng ChengDOI:10.3390/ijms22115482日期:——
A series of saccharide-modified thiadiazole sulfonamide derivatives has been designed and synthesized by the “tail approach” and evaluated for inhibitory activity against carbonic anhydrases II, IX, and XII. Most of the compounds showed high topological polar surface area (TPSA) values and excellent enzyme inhibitory activity. The impacts of some compounds on the viability of HT-29, MDA-MB-231, and MG-63 human cancer cell lines were examined under both normoxic and hypoxic conditions, and they showed certain inhibitory effects on cell viability. Moreover, it was found that the series of compounds had the ability to raise the pH of the tumor cell microenvironment. All the results proved that saccharide-modified thiadiazole sulfonamides have important research prospects for the development of CA IX inhibitors.
表征谱图
-
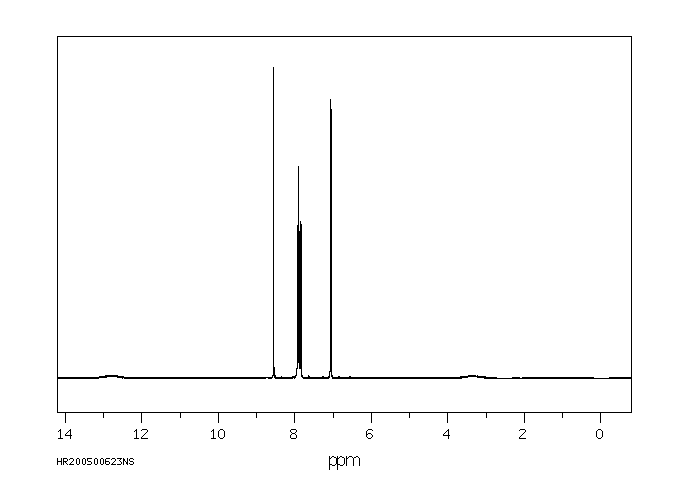
氢谱1HNMR
-
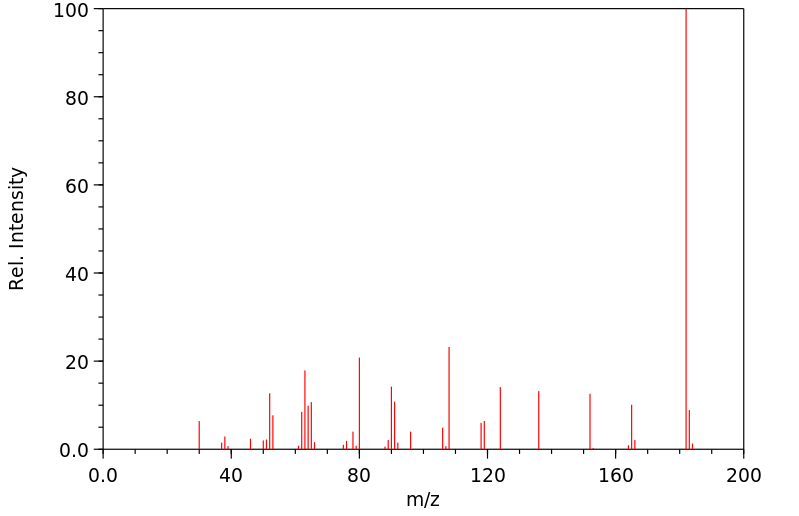
质谱MS
-
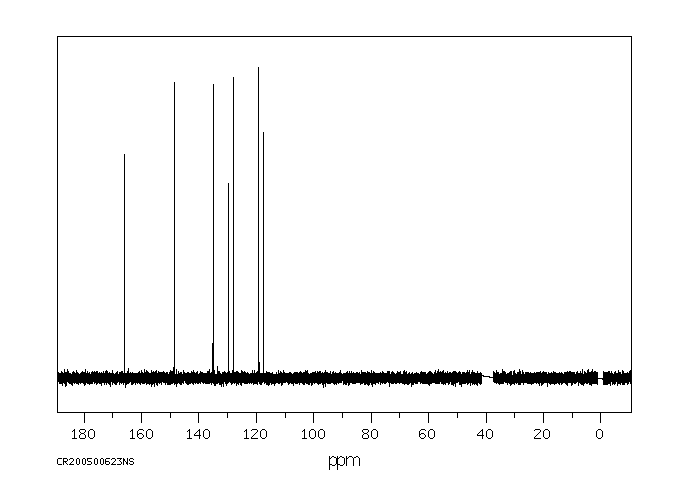
碳谱13CNMR
-
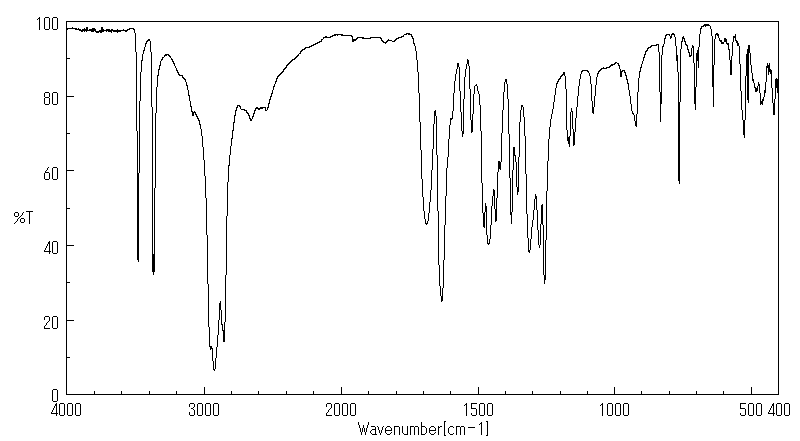
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息