N6-methyladenosine | 1867-73-8
分子结构分类
中文名称
——
中文别名
——
英文名称
N6-methyladenosine
英文别名
N-methyladenosine;6-N-methyladenosine;(6-methyl)adenosine;(2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(methylamino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol;N6-methyladenosin;N6‑methyladenosine;N6–Me-adenosine;m6A;NSC 29409;N6-Methyladenosine;(2R,3S,4R,5R)-2-(hydroxymethyl)-5-[6-(methylamino)purin-9-yl]oxolane-3,4-diol
CAS
1867-73-8
化学式
C11H15N5O4
mdl
——
分子量
281.271
InChiKey
VQAYFKKCNSOZKM-IOSLPCCCSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172 °C
-
沸点:649.1±65.0 °C(Predicted)
-
密度:1.85±0.1 g/cm3(Predicted)
-
溶解度:DMSO(轻微)、甲醇(轻微、加热、超声处理)
-
最大波长(λmax):262 (pH 1);266 (pH 7)
-
物理描述:Solid
-
碰撞截面:170.4 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:20
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.55
-
拓扑面积:126
-
氢给体数:4
-
氢受体数:8
安全信息
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
制备方法与用途
生物活性
N6-甲基腺苷(m6A、NSC-29409、6-甲基腺苷、N-甲基腺苷)是一种碱基修饰的腺苷类似物,在天然RNA中作为稀有核苷。在mRNA中,N6-甲基腺苷(m6A)是第五种碱基。
靶点Human Endogenous Metabolite
体外研究N6-甲基腺苷是一种丰富的碱基修饰的腺苷类似物,在一些病毒和大多数真核生物中被发现,包括哺乳动物、昆虫、植物和酵母。它还存在于tRNA、rRNA、小核RNA(snRNA)以及某些长链非编码RNA,如Xist中。N6-甲基腺苷是转运核糖核酸(tRNA)降解的一种内源性尿苷产物。在许多组织中广泛存在,特别是在大脑中水平较高。N6-甲基腺苷在小鼠和人类mRNAs的终止密码子附近及3’UTRs内部富集。近期研究发现FTO,一个肥胖风险基因,编码m6A去甲基酶,而m6A是调节重要生理学过程的关键因素。
体内研究LD50:小鼠灌胃大于1克/千克。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N6,N6-二甲基腺苷 N,N-dimethyladenosine 2620-62-4 C12H17N5O4 295.298 腺苷 adenosine 58-61-7 C10H13N5O4 267.244 N6-甲氧基-N6-甲基腺苷 N6-Methoxy-N6-methyladenosine 52376-60-0 C12H17N5O5 311.297 —— N6-aminoadenosine 5746-27-0 C10H14N6O4 282.259 —— 6-N-acetyl-6-N-methyladenosine 171075-26-6 C13H17N5O5 323.308 —— N6-methoxyadenosine 19399-25-8 C11H15N5O5 297.271 —— 6-azido-9-β-D-ribofuranosylpurine 53821-43-5 C10H11N7O4 293.242 —— N6-Dimethylaminomethylen-adenosin 17331-15-6 C13H18N6O4 322.324 —— N6-methyl-N6-(phenyloxycarbonyl)adenosine 161002-00-2 C18H19N5O6 401.379 2',3'-异丙叉腺苷 2',3'-isopropylidene adenosine 362-75-4 C13H17N5O4 307.309 —— N6-methyl-N6-<2-(4-nitrophenyl)ethoxycarbonyl>adenosine 161001-99-6 C20H22N6O8 474.43 —— 6-N-acetyl-2',3',5'-tris-O-(tert-butyldimethylsilyl)adenosine 171075-00-6 C30H57N5O5Si3 652.07 —— O2',O3',O5'-tris-(tert-butyl-dimethyl-silanyl)-adenosine 64911-28-0 C28H55N5O4Si3 610.032 6-氯嘌呤核苷 6-Chloropurine riboside 5399-87-1 C10H11ClN4O4 286.675 肌苷 inosine 58-63-9 C10H12N4O5 268.229 —— 6-N-acetyl-2',3',5'-tris-O-(tert-butyldimethylsilyl)-6-N-methyladenosine 171075-05-1 C31H59N5O5Si3 666.096 1-甲基腺苷酸 1-methyladenosine 15763-06-1 C11H15N5O4 281.271 2,3,5-三-O-乙酰基-6-氯嘌呤-9-β-D-呋喃核糖苷 2',3',5'-O-triacetyl-6-chloroinosine 5987-73-5 C16H17ClN4O7 412.787 6-巯嘌呤-9-beta-D-呋喃核糖苷2',3',5'-三乙酸酯 6-thio-9-(2',3',5'-tri-O-acetyl-β-D-ribosyl)purine 3021-21-4 C16H18N4O7S 410.408 2’,3’,5’-三乙酰肌苷 2',3',5'-tri-O-acetylinosine 3181-38-2 C16H18N4O8 394.341 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N6-甲基-2'-O-甲基腺苷 N6,O2'-methyladenosine 57817-83-1 C12H17N5O4 295.298 腺苷 adenosine 58-61-7 C10H13N5O4 267.244 —— N6-methyl AMP 4229-50-9 C11H16N5O7P 361.251 N6-甲基-ATP N6-Methyl-ATP 3130-39-0 C11H18N5O13P3 521.211 8-溴-N-甲基-腺苷 (2R,3R,4S,5R)-2-(8-bromo-6-(methylamino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol 37116-71-5 C11H14BrN5O4 360.167 —— 5'-O-(4,4'-dimethoxytrityl)-N6-methyladenosine 588698-71-9 C32H33N5O6 583.644 —— 1-(4-tert-butylphenyl)-3-[3-[[(2S,3S,4R,5R)-3,4-dihydroxy-5-[6-(methylamino)purin-9-yl]oxolan-2-yl]methylsulfanyl]propyl]urea 1394956-18-3 C25H35N7O4S 529.663 —— 9-[(3aR,4R,6R,6aR)-6-(aminomethyl)-2,2-dimethyl-3a,4,6,6a-tetrahydrofuro[3,4-d][1,3]dioxol-4-yl]-N-methylpurin-6-amine 1338596-01-2 C14H20N6O3 320.351 —— N6-methyl-N6-<2-(4-nitrophenyl)ethoxycarbonyl>adenosine 161001-99-6 C20H22N6O8 474.43 —— S-(((3aS,4S,6R,6aR)-2,2-dimethyl-6-(6-(methylamino)-9H-purin-9-yl)tetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)ethanethioate —— C16H21N5O4S 379.44 —— 5'-O-(4,4'-dimethoxytrityl)-N6-methyl-2'-O-[[(triisopropylsilyl)oxy]methyl]adenosine 661471-87-0 C42H55N5O7Si 770.014 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Fujii, Tozo; Saito, Tohru, Heterocycles, 1982, vol. 17, p. 117 - 123摘要:DOI:
-
作为产物:描述:参考文献:名称:High-Throughput Five Minute Microwave Accelerated Glycosylation Approach to the Synthesis of Nucleoside Libraries摘要:[GRAPHICS]The Vorbruggen glycosylation reaction was adapted into a one-step 5 min/130 degrees C microwave assisted reaction. Triethanolamine in acetontrile containing 2% water was determined to be optimal for the neutralization of trimethylsilyl triflate allowing for direct MPLC purification of the reaction mixture. When coupled with a NH3/methanol deprotection reaction, a high-throughput method of nucleoside library synthesis was enabled. The method was demonstrated by examining the ribosylation of 48 nitrogen containing heteroaromatic bases that included 25 purines, four pyrazolopyrimidines, two 8-azapurines, one 2-azapurine, two imidazopyridines, two benzimidazoles, three imidazoles, three 1,2,4-triazoles, two pyrimidines, two 3-deazapyrimidines, one quinazolinedione, and one alloxazine. Of these, 32 yielded single regioisomer products, and six resulted in separable mixtures. Seven examples provided inseparable regioisomer mixtures of -two to three compounds (16 nucleosides), and three examples failed to yield isolable products. For the 45 single isomers isolated, the average two-step overall yield +/- SD was 26 +/- 16%, and the average purity +/- SD was 95 +/- 6%. A total of 58 different nucleosides were prepared of which 15 had not previously been accessed directly from glycosylation/deprotection of a readily available base.DOI:10.1021/jo061885l
文献信息
-
[EN] BIOACTIVE CONJUGATES FOR OLIGONUCLEOTIDE DELIVERY<br/>[FR] CONJUGUÉS BIOACTIFS POUR L'ADMINISTRATION D'OLIGONUCLÉOTIDES申请人:UNIV MASSACHUSETTS公开号:WO2017030973A1公开(公告)日:2017-02-23Provided herein are self-delivering oligonucleotides that are characterized by efficient RISC entry, minimum immune response and off-target effects, efficient cellular uptake without formulation, and efficient and specific tissue distribution.本文提供的自递送寡核苷酸具有高效的RISC进入、最小的免疫反应和非靶效应、无需配方的高效细胞摄取,以及高效和特异的组织分布。
-
Noncanonical RNA Nucleosides as Molecular Fossils of an Early Earth-Generation by Prebiotic Methylations and Carbamoylations作者:Christina Schneider、Sidney Becker、Hidenori Okamura、Antony Crisp、Tynchtyk Amatov、Michael Stadlmeier、Thomas CarellDOI:10.1002/anie.201801919日期:2018.5.14prebiotic routes towards RNA. Contemporary RNA, however, is not only constructed from the four canonical nucleobases (A, C, G, and U), it also contains many chemically modified (noncanonical) bases. A still open question is whether these noncanonical bases were formed in parallel to the canonical bases (chemical origin) or later, when life demanded higher functional diversity (biological origin). Here
-
NUCLEIC ACID OF FORMULA (I): GlXmGn, OR (II): ClXmCn, IN PARTICULAR AS AN IMMUNE-STIMULATING AGENT/ADJUVANT申请人:CureVac AG公开号:US20200016264A1公开(公告)日:2020-01-16The present invention relates to a nucleic acid of the general formula (I): G l X m G n , or (II): C l X m C n , which may be modified by a lipid. The nucleic acid of the invention acts as an immune-stimulating agent inducing the innate immune response. The invention relates further to a pharmaceutical composition (in a first embodiment), each containing an immune-stimulating agent according to the invention in combination with a pharmaceutically active carrier/vehicle (and, optionally, further auxiliary substances, additives and/or further adjuvants). In another embodiment, the inventive nucleic acid is combined with at least one pharmaceutically active component, a pharmaceutically acceptable carrier/vehicle (and, optionally, further auxiliary substances, additives and/or further adjuvants). Accordingly, the present invention is directed to a vaccine, which corresponds to a pharmaceutical composition of the invention (second embodiment), wherein the pharmaceutically active component induces a specific immune response (e.g. an antigen). The present invention relates likewise to the use of a nucleic acid of the invention or a pharmaceutical composition according to the invention for the treatment of infectious diseases, autoimmune diseases, allergies or cancer diseases.本发明涉及一般式(I):G l X m G n 或(II):C l X m C n的核酸,该核酸可以通过脂质进行修饰。本发明的核酸作为免疫刺激剂,诱导先天免疫应答。本发明还涉及一种药物组合物(在第一实施例中),每种组合物包含本发明的免疫刺激剂与药用活性载体/载体(以及可选的进一步辅助物质、添加剂和/或进一步佐剂)的组合。在另一实施例中,创新的核酸与至少一种药用活性成分、药用可接受的载体/载体(以及可选的进一步辅助物质、添加剂和/或进一步佐剂)结合。因此,本发明涉及一种疫苗,该疫苗对应于本发明的药物组合物(第二实施例),其中药用活性成分诱导特异免疫应答(例如抗原)。本发明同样涉及利用本发明的核酸或药物组合物治疗传染病、自身免疫疾病、过敏或癌症疾病。
-
[EN] BINDING-SITE MODIFIED LECTINS AND USES THEREOF<br/>[FR] LECTINES DE SITE DE LIAISON MODIFIÉES ET USAGE CORRESPONDANT申请人:SMARTCELLS INC公开号:WO2010088261A1公开(公告)日:2010-08-05In one aspect, the disclosure provides cross-linked materials that include multivalent lectins with at least two binding sites for glucose, wherein the lectins include at least one covalently linked affinity ligand which is capable of competing with glucose for binding with at least one of said binding sites; and conjugates that include two or more separate affinity ligands bound to a conjugate framework, wherein the two or more affinity ligands compete with glucose for binding with the lectins at said binding sites and wherein conjugates are cross-linked within the material as a result of non-covalent interactions between lectins and affinity ligands on different conjugates. These materials are designed to release amounts of conjugate in response to desired concentrations of glucose. Depending on the end application, in various embodiments, the conjugates may also include a drug and/or a detectable label.
-
Compounds for modulating RNA interference申请人:——公开号:US20040204420A1公开(公告)日:2004-10-14The present invention pertains to compounds effective at modulating (inhibiting or enhancing) RNA interference in a cell or organism. Featured compounds are set forth and exemplified herein. Therapeutic methods and pharmaceutical compositions featuring the compounds are also provided. The invention further pertains to knock-out or knock-down cells and organisms including the compounds, and methods of analysis of gene expression profiles and proteomes.
表征谱图
-
氢谱1HNMR
-
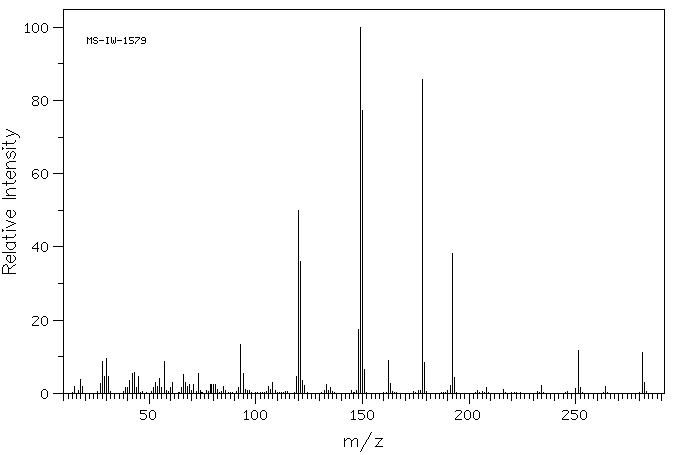
质谱MS
-
碳谱13CNMR
-
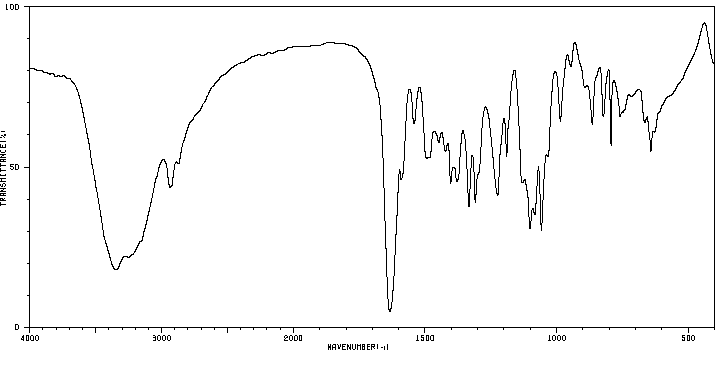
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄质核苷
黄嘌呤核苷
鸟苷5'-硫代二磷酸酯
鸟苷,N,N-二甲基-6-O-[2-(4-硝基苯基)乙基]-
鸟苷 2',3',5'-三苯甲酸酯
鸟苷
马兜铃内酰胺A
顺式-玉米素-D-核糖甙
阿糖腺苷2',3',5'-三乙酸酯
阿糖腺苷 2',3'-二乙酸酯
阿糖腺苷
阿糖腺苷
阿糖肌苷
阿洛酮糖腺苷
阿斯卡霉素
虫草素
苯酰胺,N-[9-[2-脱氧-2-氟-3,5-二-O-(四氢-2H-吡喃-2-基)-β-D-呋喃阿拉伯糖基]-9H-嘌呤-6-基]-
苯酚,4-[4-(2-氨基乙基)-2-碘苯氧基]-,盐酸(1:1)
苏云金素
腺苷酸-5-羧酸
腺苷胺类
腺苷地尔
腺苷6-N-氨基磷酸酯
腺苷5-O-硫一磷酸二锂盐
腺苷5'-O-(1-硫代二磷酸酯)
腺苷-N(6)-二乙硫基醚-N'-吡啶氧杂亚胺5'-磷酸酯
腺苷-5'-羧酸2-氯乙酯
腺苷-5'-单烟酸酯
腺苷-5'-13C
腺苷-3(+2')-单磷酸单水合物
腺苷-2,8-T2
腺苷-2'-13C
腺苷-15NN1-氧化物
腺苷,8-(丁基氨基)-N-环戊基-
腺苷,2-溴-
腺苷,2-氯-N-环丙基-
腺苷,2-[(4-甲代戊基)氧代]-
腺苷,2-(苯基甲氧基)-
腺苷,2-(环己三烯并氧基)-
腺苷,2-(2-乙基丁氧基)-
腺苷,2',3'-O-(1-甲基亚乙基)-,5'-氨基磺酸酯
腺苷
肌苷肟
肌苷-8-14C
肌苷
美他卡韦
硒-(4-硝基苯甲酰基)-6-硒肌苷
甲基4,5-二溴-3-氟噻吩-2-羧酸酯
瑞加德松杂质3
瑞加德松杂质1