phloridizin | 60-81-1
中文名称
——
中文别名
——
英文名称
phloridizin
英文别名
phlorizin;phloridzin;phloretin-2'-O-glucoside;Phlorhizin;phloretin 2′-O-glucoside;phloretin-2'-β-D-glucopyranoside;phlorizoside;phlorrhizin;1-[2,4-dihydroxy-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-3-(4-hydroxyphenyl)propan-1-one
CAS
60-81-1
化学式
C21H24O10
mdl
——
分子量
436.416
InChiKey
IOUVKUPGCMBWBT-QNDFHXLGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:113-114 °C(lit.)
-
沸点:468.89°C (rough estimate)
-
密度:1.3178 (rough estimate)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
LogP:0.452 (est)
-
物理描述:Solid
-
碰撞截面:194.4 Ų [M+Na]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:31
-
可旋转键数:7
-
环数:3.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:177
-
氢给体数:7
-
氢受体数:10
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29389090
-
RTECS号:UC2080000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:本品应密封、存于阴凉干燥处保存。
SDS
Section I.Chemical Product and Company Identification
Chemical Name Phlorizin
Portland OR
Synonym Phloridzin
Chemical Formula C21H24O10•2H2O
CAS Number 60-81-1
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
Phlorizin 60-81-1 Min. 97.0 (T) Not available. Mouse LD (intraperitoneal)
>500mg/kg
Section III. Hazards Identification
Acute Health Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Reproductive: rat (intraperitoneal) 600mg/kg. Duration: female 8 days of pregnancy.
Effects on fertility- Post-implantation mortality.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. IMMEDIATELY flush eyes with runing water for at least 15 minutes. keeping
eyelids open. COLD water may be used. DO NOT use an eye oitment. Flush eyes with running water for a minimum of
15 minutes, occasionally lifting the upper eyelids. Seek medical attention. Treat symptomatically and supportively.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thorough wash the contaminated skin with
running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. COLD water
may be used. Cover the irritated skin with an emollient. Seek medical attention. Treat symptomatically and supportively.
Wash any contaminated clothing before reusing.
Inhalation If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material
was ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible,
show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability May be combustible at high temperature.
Flash Points Flammable Limits Not available.
Not available.
Combustion Products
These products are toxic carbon oxides (CO, CO 2).
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Continued on Next Page
Phlorizin
Section VI. Accidental Release Measures
Spill Cleanup Irritating material.
Instructions In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and exercise caution. Use a
shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
IRRITANT. Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use, tightly seal the
Handling and Storage
container and store in a dry, cool place. Avoid excessive heat and light. DO NOT breathe dust.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Boots. Gloves. Be sure to use a MSHA/NIOSH approved respirator or
equivalent. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Yellow powder. 1g dissolves in water @ 22°C, 64mL water
@ 60°C, 22mL water @ 70°C.
Not available. Freely soluble in boiling water, in about 4
Specific Gravity
parts alcohol, methanol, amyl alcohol,
acetone, ethyl acetate, pyridine, aniline.
Practically insoluble in ether, chloroform
and benzene.
Molecular Weight Partition Coefficient
436.41 (Anh) Not available.
Boiling Point Vapor Pressure
Not available. Not available.
Melting Point 110°C (230°F) Vapor Density Not available.
Refractive Index Not available. Volatility Not available.
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Sweet with biter aftertaste.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with strong oxidizing agents.
Section XI. Toxicological Information
RTECS Number UC2080000
Eye contact. Ingestion. Inhalation. Skin contact.
Routes of Exposure
Toxicity Data Mouse LD (intraperitoneal) >500mg/kg
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Reproductive: rat (intraperitoneal) 600mg/kg. Duration: female 8 days of pregnancy.
Effects on fertility- Post-implantation mortality.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
Continued on Next Page
Phlorizin
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with
Waste Disposal
a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
Not applicable.
PIN Number
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) 200-487-1
EEC Risk Statements R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
根皮苷概述
根皮苷是根皮素的葡萄糖苷,化学名称为1-(2-(β-D-吡喃葡萄糖氧基)-4,6-二羟基苯基)-3-(4-羟基苯基)丙酮,属于黄酮类中的二氢查尔酮类物质。主要存在于苹果树的根皮、茎和嫩叶以及苹果果实中,在菊科、杜鹃花科、豆科、壳斗科、百合科等植物中也有少量存在。根皮苷具有降低血糖、改善记忆力、抗过敏、抗癌等多种重要的生物活性,因此在食品、美容和保健品行业都有潜在的利用价值。
分布根皮苷主要存在于蔷薇科苹果属植物中,在菊科、杜鹃花科、豆科、壳斗科、百合科等植物中也有少量存在。近年来,在荔枝果皮、杜梨叶、锁阳等植物中也发现了根皮苷的存在,但含量较少。甜茶多穗柯中有大量的根皮苷。总的来说,苹果属植物是根皮苷的主要来源,可以作为提取根皮苷的原料加以利用。苹果树枝、叶、树皮等中都含有大量的根皮苷。
提取方法 提取步骤- 材料准备:选取合适的苹果组织,如果实、茎或叶子。
- 粉碎处理:将收集的苹果材料进行研磨处理,以提高后续提取效率。
- 溶剂选择:通常采用乙醇作为溶剂,因其能有效溶解根皮苷。
- 超声波辅助提取:利用超声波技术加速提取过程,并确保化合物均匀浸出。
- 过滤与浓缩:将提取液进行过滤去除残渣后,通过减压浓缩得到粗提物。
- 纯化分离:使用柱色谱等方法对粗提物进一步纯化,获得高纯度的根皮苷。
研究表明,根皮苷具有降低空腹血糖的作用。其机制在于竞争性抑制葡萄糖转运载体(SGLTs和GLUTs)对葡萄糖分子的运输。
抗氧化作用对于由高脂膳食引起的氧化损伤,根皮苷能明显保护果蝇,并显著延长其寿命,表现出较强的抗氧化能力。
与植物生理相关性根皮苷与植物生长发育以及抗逆性等生理现象紧密相连。它对抗多种病原菌,如苹果黑星病和火疫病,但对某些植物的生长具有抑制作用。低浓度下能促进平邑甜茶幼苗生长,而高浓度则可能引发植株重茬障碍。
其他活性 应用 食品行业 医药领域 美容行业上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异杞柳甙 2',4',6',4-tetrahydroxychalcone 2'-O-β-D-glucoside 4547-85-7 C21H22O10 434.4 —— 7-hydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-5-yl β-D-glucopyranoside 23711-00-4 C21H22O10 434.4 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2',4,6'-trihydroxy-4'-methoxydihydrochalcone 2'-O-β-D-glucopyranoside 11075-15-3 C22H26O10 450.442 —— davidioside 23140-78-5 C21H24O9 420.416 —— ((2R,3S,4S,5R,6S)-6-{3,5-dihydroxy-2-(3-(4-hydroxyphenyl)propanoyl)phenoxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl dodecanoate —— C33H46O11 618.722 —— ((2R,3S,4S,5R,6S)-6-{3,5-dihydroxy-2-(3-(4-hydroxyphenyl)propanoyl)phenoxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl stearate 1415230-59-9 C39H58O11 702.883 —— (6-{3,5-dihydroxy-2-[3-(4-hydroxyphenyl)propanoyl]phenoxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl (Z)-9-octadecenoate 1421589-31-2 C39H56O11 700.867 —— (6-{3,5-dihydroxy-2-[3-(4-hydroxyphenyl)propanoyl]phenoxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl (5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-icosapentaenoate 1421589-34-5 C41H52O11 720.857 —— (6-{3,5-dihydroxy-2-[3-(4-hydroxyphenyl)propanoyl]phenoxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl (4Z,7Z,10Z,13Z,16Z,19Z)-4,7,10,13,16,19-docosahexaenoate 1421589-35-6 C43H54O11 746.895 —— (6-{3,5-dihydroxy-2-[3-(4-hydroxyphenyl)propanoyl]phenoxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl (9Z,12Z)-9,12-octadecadienoate 1421589-32-3 C39H54O11 698.851 —— (6-{3,5-dihydroxy-2-[3-(4-hydroxyphenyl)propanoyl]phenoxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl (9Z,12Z,15Z)-9,12,15-octadecatrienoate 1421589-33-4 C39H52O11 696.835 —— 3-(4-hydroxyphenyl)-2',4',6'-trihydroxypropyl 2'-O-β-D-glucopyranoside 1133352-85-8 C21H26O9 422.432 —— methylenebisphloridzin —— C43H48O20 884.842 1-(3-BETA-D-吡喃葡萄糖基-2,4,6-三羟基苯基)-3-(4-羟基苯基)-1-丙酮 nothofagin 11023-94-2 C21H24O10 436.416 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:天然查耳酮在人类巨噬细胞中引发专门的促溶解介质和相关 15-脂氧合酶产物的形成摘要:专门的促分解介体 (SPM) 包括由多不饱和脂肪酸 (PUFA) 通过立体选择性氧化作用特别是涉及 12/15-脂氧合酶 (LOX) 产生的脂质介体 (LM)。与促炎 LM(例如由 5-LOX 形成的白三烯和由环氧合酶形成的前列腺素)相比,SPM 具有抗炎和消炎特性。尽管糖皮质激素和阻止前列腺素产生的非甾体抗炎药 (NSAID) 仍然是炎症相关疾病的主要治疗方法,尽管有严重的副作用,但新概念集中在 SPM 作为抗炎药物治疗的免疫溶解剂。在这里,我们研究了来自Melodorum fruticosum 的天然查耳酮 MF-14 和相应的二氢查耳酮 MF-15,用于调节人单核细胞来源的巨噬细胞中 LM 的生物合成,包括白三烯、前列腺素、SPM 及其 12/15-LOX 衍生前体( MDM) M1 和 M2 样表型。在受到金黄色葡萄球菌衍生的外毒素攻击的 MDM 中,两种化合物 (10 µM) 均显着抑制DOI:10.1016/j.bcp.2021.114825
-
作为产物:描述:参考文献:名称:Zemplen et al., Chemische Berichte, 1943, vol. 76, p. 386,388,摘要:DOI:
-
作为试剂:描述:矢车菊素-3-O-半乳糖苷 、 乙醛 、 表儿茶素 在 苹果酸 、 (-)-5-咖啡酰奎宁酸 、 phloridizin 作用下, 以 乙醇 、 水 为溶剂, 反应 1440.0h, 生成 2-(3,4-Dihydroxy-phenyl)-8-{1-[(2R,3R)-2-(3,4-dihydroxy-phenyl)-3,6,7-trihydroxy-chroman-8-yl]-ethyl}-5,7-dihydroxy-3-((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxy)-chromenylium参考文献:名称:Characterization and structures of anthocyanin pigments generated in rosé cider during vinification摘要:Anthocyanin pigments, which are trot found in apple juice, were detected in rose cider. We confirmed by HPLC/DAD and LC/ESI-MS analyses that some of these anthocyanin pigments generated in rose cider during vinification corresponded to those formed in model cider containing anthocyanin and flavan-3-ol in the presence of acetaldehyde. To confirm their structures, two anthocyanin pigments formed in a model cider containing cyanidin-3-galactoside and (-)-epicatechin in the presence of acetaldehyde were isolated and purified, and their structures were elucidated by high resolution FAB-MS and H-1 and C-13 NMR analyses. These two pigments were found to consist of cyanidin-3-galactoside and (-)-epicatechin linked by a CH3-CH bridge at the 8-position. They were diastereomers that differed in the configuration of the asymmetric methine carbon. (C) 2002 Published by Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0031-9422(01)00427-7
文献信息
-
NOVEL GLUCOKINASE ACTIVATORS AND METHODS OF USING SAME申请人:Ryono Denis E.公开号:US20080009465A1公开(公告)日:2008-01-10Compounds are provided which are phosphonate and phosphinate activators and thus are useful in treating diabetes and related diseases and have the structure wherein is a heteroaryl ring; R 4 is —(CH 2 ) n -Z-(CH 2 ) m —PO(OR 7 )(OR 8 ), —(CH 2 ) n Z-(CH 2 ) m —PO(OR 7 )R g , —(CH 2 ) n -Z-(CH 2 ) m —OPO(OR 7 )R g , —(CH 2 ) n Z—(CH 2 ) m —OPO(R 9 )(R 10 ), or —(CH 2 ) n Z—(CH 2 ) m —PO(R 9 )(R 10 ); R 5 and R 6 are independently selected from H, alkyl and halogen; Y is R 7 (CH 2 ) s or is absent; and X, n, Z, m, R 4 , R 5 , R 6 , R 7 , and s are as defined herein; or a pharmaceutically acceptable salt thereof. A method for treating diabetes and related diseases employing the above compounds is also provided.提供了磷酸酯和磷酸酯激活剂,因此在治疗糖尿病和相关疾病方面非常有用,并具有以下结构: 其中 是杂环芳基环; R 4 为—(CH 2 ) n -Z-(CH 2 ) m —PO(OR 7 )(OR 8 )、—(CH 2 ) n Z-(CH 2 ) m —PO(OR 7 )R g 、—(CH 2 ) n -Z-(CH 2 ) m —OPO(OR 7 )R g 、—(CH 2 ) n Z—(CH 2 ) m —OPO(R 9 )(R 10) 或—(CH 2 ) n Z—(CH 2 ) m —PO(R 9 )(R 10) ; R 5 和R 6 分别选择自H、烷基和卤素; Y为R 7 (CH 2 ) s 或不存在;以及 X、n、Z、m、R 4 、R 5 、R 6 、R 7 和s如本文所定义;或其药用盐。 还提供了一种利用上述化合物治疗糖尿病和相关疾病的方法。
-
3-AMINOIMIDAZO 1,2-A PYRIDINE DERIVATIVES HAVING AN SGLT1- AND SGLT2-INHIBITING ACTION FOR THE TREATMENT OF TYPE 1 AND TYPE 2 DIABETES申请人:Klein Markus公开号:US20100305142A1公开(公告)日:2010-12-02Novel compounds of the formula (I), in which W, T, R 1 , R 2 , R 3 , R 4 , R 5 and R 6 have the meanings indicated in Patent Claim ( 1 ), are suitable as antidiabetics.化合物的新颖结构如下(I)式,其中W,T,R1,R2,R3,R4,R5和R6的含义如专利权要求(1)中所示,适用作为抗糖尿病药物。
-
ARYL GLYCOSIDE COMPOUND, PREPARATION METHOD AND USE THEREOF申请人:Xu Zusheng公开号:US20130324464A1公开(公告)日:2013-12-05Disclosed are an aryl glycoside compound as represented by formula I or formular I′, a pharmaceutically acceptable salt thereof, optical isomer thereof or a prodrug thereof. The present invention relates to a method of preparing said aryl glycoside compound and the use thereof The aryl glycoside compound of the present invention has an excellent ability on inhibit SGLT activity, especially SGLT2 activity, and is diabetes-fighting medicine with great potential.
-
Benzimidazole Derivatives申请人:Burgdorf Lars Thore公开号:US20100016372A1公开(公告)日:2010-01-21Novel compounds of the formula I (I), in which R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R, Q, W, X and Z have the meanings indicated in Patent Claim 1 , are suitable as antidiabetics.公式I(I)的新化合物,其中R1,R2,R3,R4,R5,R6,R7,R8,R9,R10,R11,R12,R13,R,Q,W,X和Z的含义如专利权要求书中所示,适用于抗糖尿病药物。
-
IMIDAZO 1, 2-A PYRIMIDINE DERIVATIVES FOR THE TREATMENT OF DISEASES SUCH AS DIABETES申请人:Klein Markus公开号:US20100249151A1公开(公告)日:2010-09-30Novel compounds of the formula (I), in which W, T, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 and X 7 have the meanings indicated in Patent Claim 1 , are suitable as antidiabetics.化合物的新颖结构式(I),其中W、T、R1、R2、R3、R4、R5、R6和X7的含义如专利权要求书中所示,适用于抗糖尿病药物。
表征谱图
-
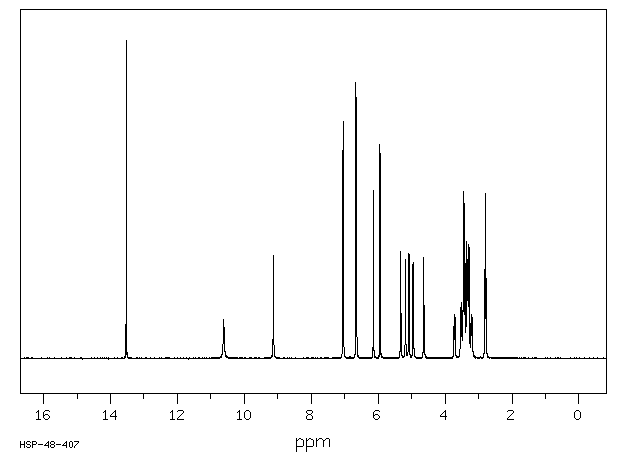
氢谱1HNMR
-
质谱MS
-
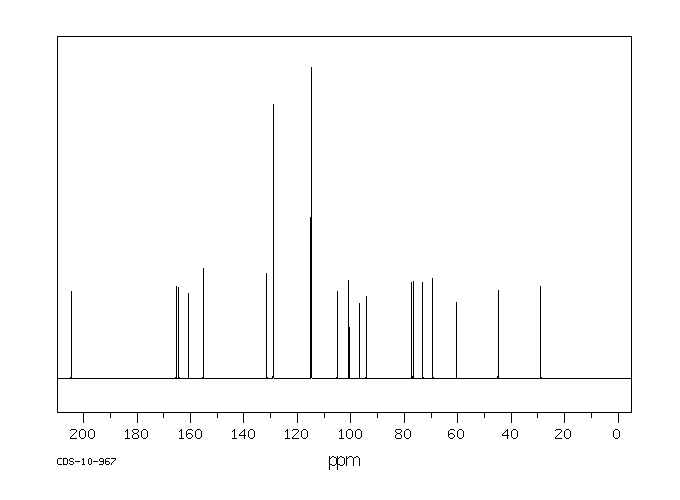
碳谱13CNMR
-
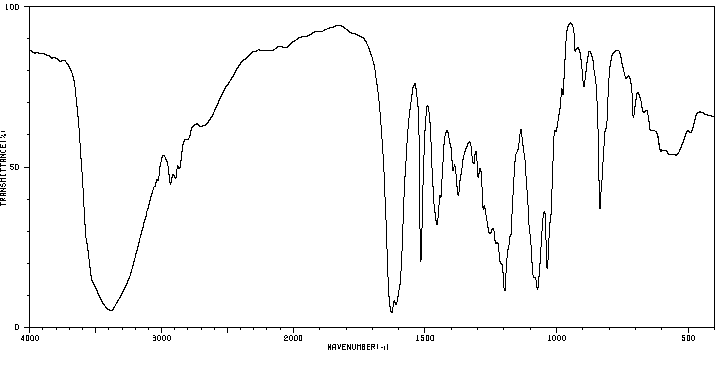
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷