5-氨基异喹啉 | 1125-60-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:125-128 °C (lit.)
-
沸点:312.78°C (estimate)
-
密度:1.1148 (estimate)
-
溶解度:可溶于氯仿、乙酸乙酯、甲醇
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也没有已知的危险反应。应避免与氧化物或光线接触。
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:38.9
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36/37/39,S37/39
-
危险类别码:R20/21/22,R38
-
WGK Germany:3
-
海关编码:2933499090
-
危险品运输编号:25kgs
-
包装等级:III
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
: 5-氨基异喹啉
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H8N2
分子式
: 144.17 g/mol
分子量
组分 浓度或浓度范围
Isoquinol-5-ylamine
-
CAS 号 1125-60-6
EC-编号 214-408-3
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 黄色, 棕色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 125 - 128 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-硝基异喹啉 5-nitroisoquinoline 607-32-9 C9H6N2O2 174.159 5-氨基-1-氯异喹啉 1-chloroisoquinolin-5-amine 374554-54-8 C9H7ClN2 178.621 异喹啉 isoquinoline 119-65-3 C9H7N 129.161 —— 5-nitroisoquinoline N-oxide 57554-78-6 C9H6N2O3 190.158 异喹啉-N-氧化物 Isoquinoline N-oxide 1532-72-5 C9H7NO 145.161 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5,6-异喹啉二胺 5,6-diaminoisoquinoline 140192-89-8 C9H9N3 159.191 5-肼基异喹啉 5-hydrazinylisoquinoline 90564-61-7 C9H9N3 159.191 5,8-二氨基异喹啉 5,8-diaminoisoquinoline 1127-49-7 C9H9N3 159.191 8-氨基异喹啉 8-aminoisoquinoline 23687-27-6 C9H8N2 144.176 5-叠氮基异喹啉 5-azidoisoquinoline 43101-10-6 C9H6N4 170.173 (9ci)-5-异硫代氰酰基异喹啉 5-isothiocyanatoisoquinoline 62855-11-2 C10H6N2S 186.237 5-异氰酸异喹啉 5-isocyanatoisoquinoline 581812-66-0 C10H6N2O 170.17 —— N-(5-isoquinolinyl)thiourea 72677-72-6 C10H9N3S 203.268 5-乙酰氨基异喹啉 N-(isoquinolin-5-yl)acetamide 27461-33-2 C11H10N2O 186.213 8-溴异喹啉-5-胺 8-bromoisoquinolin-5-amine 90721-34-9 C9H7BrN2 223.072 —— N-(5-isoquinolinyl)propionamide 1190561-61-5 C12H12N2O 200.24 —— 5-(N'-methylthioureido)isoquinoline 453527-47-4 C11H11N3S 217.294 —— 1-(isoquinolin-5-yl)-3-phenylurea 119612-64-5 C16H13N3O 263.299 —— methyl isoquinoline-5-carbamate 140192-77-4 C11H10N2O2 202.213 —— 2-chloro-N-(isoquinolin-5-yl)acetamide 16880-59-4 C11H9ClN2O 220.658 1-异喹啉-5-基-3-苯基硫脲 N-phenyl-N1-(5-isoquinolinyl)thiourea 119612-67-8 C16H13N3S 279.365 —— N-[8-(hexyloxy)octyl]isoquinolin-5-amine —— C23H36N2O 356.552 —— N-(isoquinolin-5-yl)-2-methylpropanamide 1202995-95-6 C13H14N2O 214.267 异喹啉 isoquinoline 119-65-3 C9H7N 129.161 —— N-ethyl-N1-(5-isoquinolinyl)thiourea 140192-80-9 C12H13N3S 231.321 —— 3-bromo-N-(5-isoquinolyl)propanamide —— C12H11BrN2O 279.136 —— ethyl isoquinoline-5-carbamate 140192-78-5 C12H12N2O2 216.239 —— N-(5-isoquinolinamino)-2-iminopropionic acid 6502-57-4 C12H11N3O2 229.238 —— 5-trifluoroacetamidoisoquinoline 14818-60-1 C11H7F3N2O 240.185 —— 2,2,2-trichloro-N-(isoquinolin-5-yl)acetamide 581812-64-8 C11H7Cl3N2O 289.548 —— 5-amino-6,8-dibromoisoquinoline 90224-88-7 C9H6Br2N2 301.968 —— 1-Isoquinolin-5-yl-3-propylthiourea 1095395-34-8 C13H15N3S 245.348 —— 1-(isoquinolin-5-yl)-3-p-tolylthiourea 437729-52-7 C17H15N3S 293.392 —— 1-(4-Fluorophenyl)-3-(isoquinolin-5-yl)thiourea —— C16H12FN3S 297.356 —— AC1M6XNM —— C16H12ClN3S 313.81 —— N-(pyrrolidin-3-yl)isoquinolin-5-amine 1035096-80-0 C13H15N3 213.282 —— 4-(5-isoquinolylamino)-1-cyclohexanone 353561-85-0 C15H16N2O 240.305 —— (R)-N-(pyrrolidin-3-yl)isoquinolin-5-amine 1035096-91-3 C13H15N3 213.282 —— 5-acetamido-6-aminoisoquinoline 140192-86-5 C11H11N3O 201.228 —— N-(5-isoquinolyl)-N-(1-propyl-4-piperidyl)-amine —— C17H23N3 269.39 —— N-5-isoquinolinyl-N'-pentylurea 581810-64-2 C15H19N3O 257.335 8-硝基-5-异喹啉胺 5-amino-8-nitroisoquinoline 156901-58-5 C9H7N3O2 189.173 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:[EN] THERAPEUTIC METHODS AND COMPOUNDS
[FR] PROCÉDÉS ET COMPOSÉS THÉRAPEUTIQUES摘要:该发明提供了一种化合物的公式I:(I)或其药用可接受的盐,其中R1-R5 Y具有规范中描述的任何值,以及包含公式I化合物的组合物。这些化合物可用于治疗疟疾。公开号:WO2020219591A1 -
作为产物:参考文献:名称:可见光下非均相 V2O5/TiO2 介导的硝基化合物光催化还原为相应的胺摘要:在环境温度下,在蓝色 LED (9 W) 照射下,使用非均相且可回收的催化剂 (V 2 O 5 /TiO 2 ) 将硝基化合物氢化成相应的胺。采用水合肼作为还原剂,乙醇作为溶剂,促进绿色、可持续、低成本生产。描述了 32 种(杂)芳胺及其药学相关分子(五种)的合成。该协议的显着特点包括催化剂可回收性、绿色溶剂、环境温度和克级反应。研究的其他方面包括1H-NMR 辅助反应进程监测、机理研究的控制实验、方案应用和可回收性研究。此外,所开发的方案实现了广泛的官能团耐受性、化学选择性、高产率、低成本、可持续且环境友好的合成。DOI:10.1021/acs.joc.3c00569
-
作为试剂:描述:5-氨基异喹啉 、 三氟乙酸 、 1-叔丁氧碳基-3-吡咯烷酮 、 三乙酰氧基硼氢化钠 在 氮 、 5-氨基异喹啉 、 sodium hydroxide 、 醋酸异丙酯 、 水 、 甲基叔丁基醚 作用下, 以 四氢呋喃 为溶剂, 反应 6.83h, 以Approximately 536 g of tert-Butyl 3-(isoquinolin-5-ylamino)pyrrolidine-1-carboxylate was isolated as a solid (82% yield)的产率得到3-(isoquinolin-5-ylamino)pyrrolidine-1-carboxylic acid tert-butyl ester参考文献:名称:PROCESS FOR THE PREPARATION OF RHO-KINASE INHIBITOR COMPOUNDS摘要:本发明涉及实用的高产率合成过程,用于制备通式III、IV、V、VII、VIII、IX、X、XII、XIV和XV的化合物。这些化合物可用作最终产品,也可用作中间体,并进一步改性以制备其他所需产品,如rho-激酶抑制剂。本发明还涉及某些新颖化合物和/或某些化合物的新颖固态形式。公开号:US20100022775A1
文献信息
-
SUBSTITUTED BRIDGED UREA ANALOGS AS SIRTUIN MODULATORS申请人:GLAXOSMITHKLINE LLC公开号:US20150152108A1公开(公告)日:2015-06-04The present invention relates to novel substituted bridged urea compounds, corresponding related analogs, pharmaceutical compositions and methods of use thereof. Sirtuin-modulating compounds of the present invention may be used for increasing the lifespan of a cell, and treating and/or preventing a wide variety of diseases and disorders, which include, but are not limited to, for example, diseases or disorders related to aging or stress, diabetes, obesity, neurodegenerative diseases, cardiovascular disease, blood clotting disorders, inflammation, cancer, and/or flushing as well as diseases or disorders that would benefit from increased mitochondrial activity. The present invention also related to compositions comprising a sirtuin-modulating compound in combination with another therapeutic agent.本发明涉及新型取代桥式脲化合物,相应的相关类似物,药物组合物以及其使用方法。本发明的抑制素调节化合物可用于延长细胞寿命,并治疗和/或预防各种疾病和疾病,包括但不限于与衰老或压力、糖尿病、肥胖、神经退行性疾病、心血管疾病、血液凝块疾病、炎症、癌症和/或潮红有关的疾病或疾病,以及那些会受益于增加线粒体活性的疾病或疾病。本发明还涉及包含抑制素调节化合物与另一治疗剂组合的组合物。
-
[EN] SUBSTITUTED BRIDGED UREA ANALOGS AS SIRTUIN MODULATORS<br/>[FR] ANALOGUES D'URÉE PONTÉS SUBSTITUÉS EN TANT QUE MODULATEURS DE SIRTUINE申请人:GLAXOSMITHKLINE IP NO 2 LTD公开号:WO2016079709A1公开(公告)日:2016-05-26The present invention relates to novel substituted bridged urea analog compounds of Formula (I) or pharmaceutically acceptable salts thereof, corresponding pharmaceutical compositions, processes for making and use of such compounds, alone or in combination with other therapeutic agents, as Sirtuin Modulators useful for increasing lifespan of a cell, and for use in treating and/or preventing a wide variety of diseases and disorders, which include, but are not limited to, for example, diseases or disorders related to aging or stress, diabetes, obesity, neurodegenerative diseases, cardiovascular disease, blood clotting disorders, inflammation, cancer, and/or flushing as well as diseases or disorders that would benefit from increased mitochondrial activity.
-
Vanilloid receptor ligands and their use in treatments申请人:Gore Keshav Vijay公开号:US20060084640A1公开(公告)日:2006-04-20Therapeutic benzimidazoles and compositions containing them, for the treatment of acute, inflammatory and neuropathic pain, dental pain, general headache, migraine, cluster headache, mixed-vascular and non-vascular syndromes, tension headache, general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, inflammatory pain and associated hyperalgesia and allodynia, neuropathic pain and associated hyperalgesia and allodynia, diabetic neuropathy pain, causalgia, sympathetically maintained pain, deafferentation syndromes, asthma, epithelial tissue damage or dysfunction, herpes simplex, disturbances of visceral motility at respiratory, genitourinary, gastrointestinal or vascular regions, wounds, burns, allergic skin reactions, pruritus, vitiligo, general gastrointestinal disorders, gastric ulceration, duodenal ulcers, diarrhea, gastric lesions induced by necrotising agents, hair growth, vasomotor or allergic rhinitis, bronchial disorders or bladder disorders.治疗性苯并咪唑及含有它们的组合物,用于治疗急性、炎症性和神经痛、牙痛、普通头痛、偏头痛、集群头痛、混合血管和非血管综合征、紧张性头痛、一般炎症、关节炎、风湿性疾病、骨关节炎、炎症性肠道疾病、炎症性眼部疾病、炎症性或不稳定的膀胱疾病、牛皮癣、带有炎症成分的皮肤疾病、慢性炎症症状、炎症性疼痛及相关的过敏性疼痛和触痛、神经痛及相关的过敏性疼痛和触痛、糖尿病性神经病痛、烧灼性疼痛、交感神经维持性疼痛、去神经症候群、哮喘、上皮组织损伤或功能障碍、单纯疱疹、呼吸、泌尿、消化或血管区域内脏运动障碍、伤口、烧伤、过敏性皮肤反应、瘙痒、白癜风、一般胃肠道疾病、胃溃疡、十二指肠溃疡、腹泻、由坏死性剂引起的胃病变、毛发生长、血管运动性或过敏性鼻炎、支气管疾病或膀胱疾病。
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。 -
[EN] ANTI-CANCER AND ANTI-HIV COMPOUNDS<br/>[FR] COMPOSÉS ANTICANCÉREUX ET ANTI-VIH申请人:SIRENAS MARINE DISCOVERY公开号:WO2014123900A1公开(公告)日:2014-08-14Disclosed herein are compounds useful as anti-cancer and anti-HIV agents. Also disclosed are pharmaceutical compositions and methods of treatment. The compounds disclosed herein can be used to treat a variety of conditions, diseases and ailments such as bladder cancer, breast cancer, colon cancer, rectal cancer, endometrial cancer, kidney cancer, lung cancer, melanoma, non-Hodgkin lymphoma, glioblastoma, pancreatic cancer, prostate cancer, and thyroid cancer, and HIV related disorders.
表征谱图
-
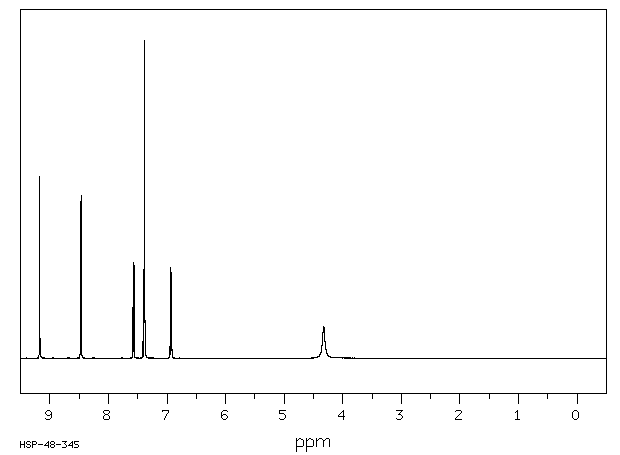
氢谱1HNMR
-
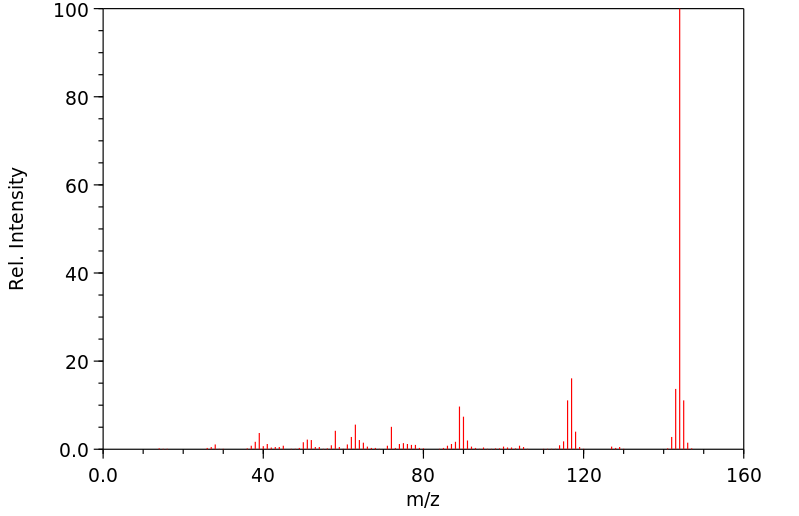
质谱MS
-
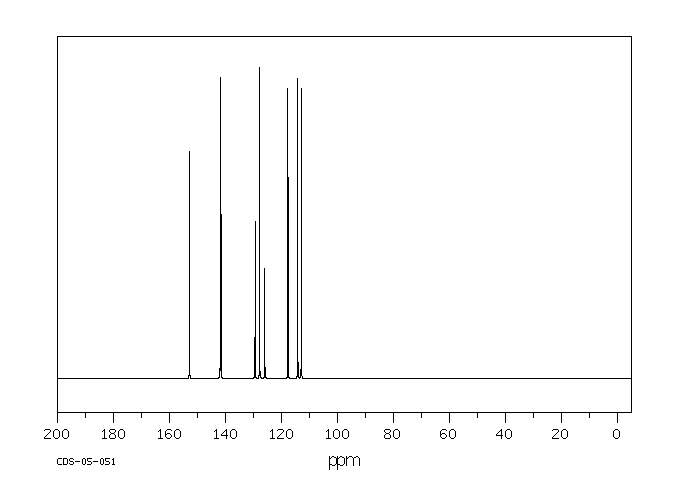
碳谱13CNMR
-
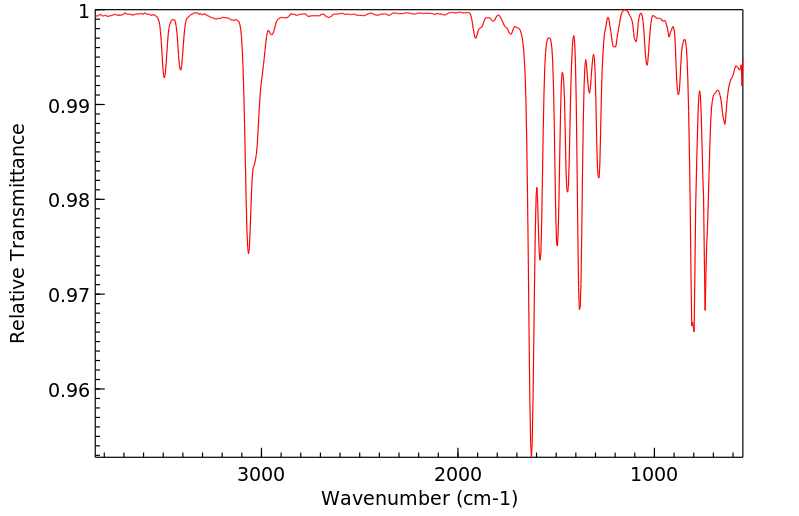
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息