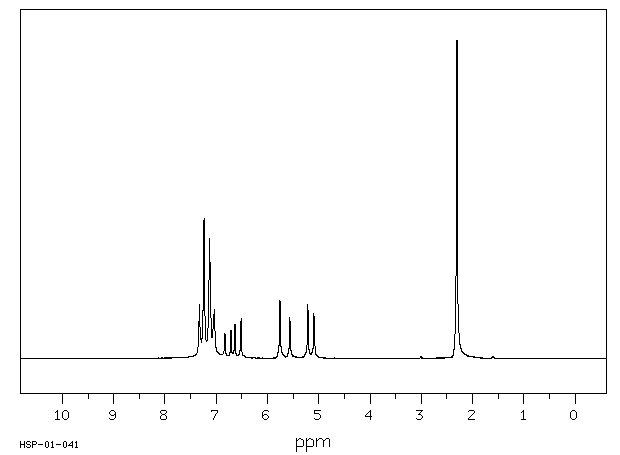
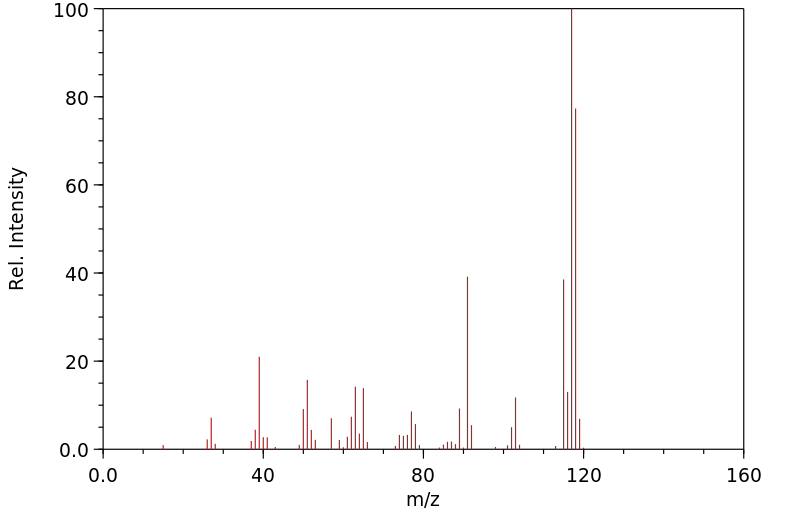
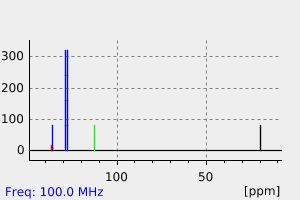
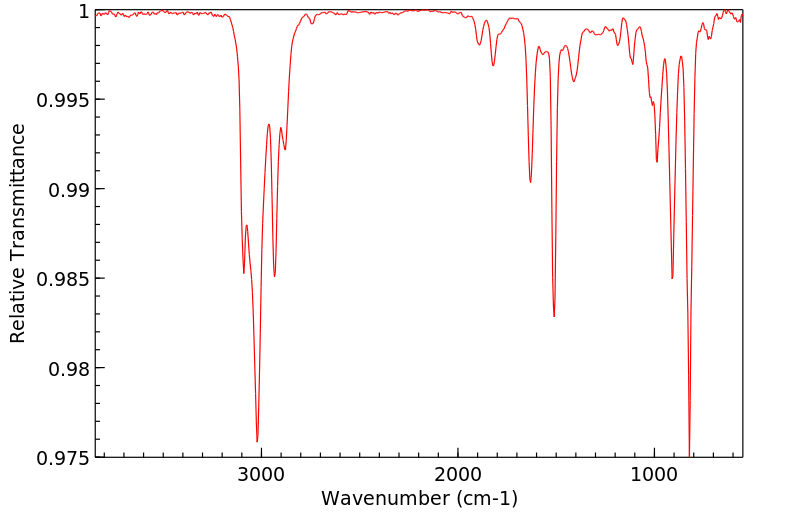
代谢
o-乙烯基甲苯、m-乙烯基甲苯和p-乙烯基甲苯通过腹腔注射给大鼠,并通过气相色谱质谱法分析尿液中的酸性代谢物。制备了不同的衍生物,用于识别代谢物的结构。发现了不同类型(11种)的代谢物,尽管不是所有的代谢物都是由每种乙烯基甲苯同分异构体产生的。识别出的代谢物包括:甲基苯乙烯乙二醇、甲基马兰酸、甲基苯基乙二酸、羟基甲基苯乙烯乙二醇、羧基苯乙烯乙二醇、羟基甲基苯基乙二酸、甲基苯甲酰甘氨酸、甲基苯乙酰甘氨酸、乙烯基苯甲酰甘氨酸、甲基苯乙醇的N-乙酰半胱氨酸结合物和甲基苯乙烯乙二醇的葡萄糖苷酸。回收的代谢物(>90%)在最初的24小时内排出。对于所有代谢物的总和,给予剂量与回收代谢物量之间大约呈线性关系。重复每日给药并未导致代谢物排泄的显著增加,也没有明显的酶诱导现象。
o-Vinyltoluene, m-vinyltoluene and p-vinyltoluene were given i.p. to rats and urine was analyzed for acidic metabolites by gas chromatography mass spectrometry. Different derivatives were prepared and used to identify the structure of the metabolites. Different types (11) of metabolites were found, although all metabolites were not produced from each vinyltoluene isomer. The metabolites identified were: methylphenylethylene glycol, methylmandelic acid, methylphenylglyoxylic acid, hydroxymethylphenylethylene glycol, carboxyphenylethylene glycol, hydroxymethylphenylglyoxylic acid, methylbenzoylglycine, methylphenylacetylglycine, vinylbenzoylglycine, N-acetylcysteine conjugate of methylphenylethanol and glucuronide of methylphenylethylene glycol. Metabolites (> 90%) recovered were excreted during the 1st 24 hr. For the sum of all metabolites, there was an approximately linear relation between given dose and recovered amount of metabolites. Repeated daily administration was not followed by any appreciable increase in metabolite excretion and no enzyme induction phenomenon was apparent.
来源:Hazardous Substances Data Bank (HSDB)