N-乙基苯胺 | 103-69-5
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-63 °C
-
沸点:205 °C(lit.)
-
密度:0.963 g/mL at 25 °C(lit.)
-
蒸气密度:4.2 (vs air)
-
闪点:185 °F
-
溶解度:2.7g/l
-
介电常数:5.9(20℃)
-
暴露限值:NIOSH: IDLH 100 ppm
-
LogP:2.26 at 25℃ and pH6-8
-
物理描述:N-ethylaniline appears as a dark liquid with an aromatic odor. Insoluble in water. Density 0.963 g / cm3. Toxic by skin absorption and inhalation of vapors. Evolves toxic fumes during combustion. Flash point 185°F.
-
颜色/状态:Colorless liquid
-
气味:Aniline-like odor
-
蒸汽密度:4.18 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.204 mm Hg @ 25 °C
-
亨利常数:9.78e-06 atm-m3/mole
-
稳定性/保质期:
-
自燃温度:480 °C
-
分解:Decomposes on contact with air or light.
-
粘度:1.716X10-2 Pa.s
-
汽化热:6.57X10+7 J/kmol @ 209.65 K
-
表面张力:4.579X10-2 N.m @ 209.65 K
-
折光率:Very refractive; Index of refraction: 1.5559 @ 20 °C/D
-
解离常数:pKa= 5.12 at 25 °C (conjugate acid)
-
保留指数:1100;1125;1111.5;1100
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:9
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S28,S28A,S37,S45
-
危险类别码:R23/24/25,R33
-
WGK Germany:1
-
海关编码:2921499090
-
危险品运输编号:UN 2272 6.1/PG 3
-
危险类别:6.1
-
RTECS号:BX9780000
-
包装等级:III
-
储存条件:储存注意事项:应存放在阴凉、通风的库房中,远离火种与热源。包装需密封,避免与空气接触。应与其他化学品分开存放,尤其是要与氧化剂、酸类及食用化学品隔开,严禁混储。同时,须配备相应种类和数量的消防设备。储存区域还需备有泄漏应急处理工具以及适合的吸收材料。
制备方法与用途
化学性质
无色液体。熔点为-63.5℃(凝固点为-80℃),沸点204.5℃,在1.33kPa压力下的沸点为83.8℃,相对密度为0.958(25℃)和0.9625(20℃),折射率为1.5559,闪点与燃点均为85℃。它不溶于水和乙醚,但可溶于醇及大多数有机溶剂。见光或暴露在空气中会迅速变褐色,并散发苯胺气味。
用途
该产品广泛用于有机合成,是偶氮染料和三苯甲烷染料的重要中间体;还用作橡胶助剂、炸药、照相材料等精细化学品的中间体。此外,它也可作为农药及染料中间体、橡胶促进剂等。
生产方法
- 盐酸法:在180℃和2.94MPa的压力下,苯胺盐酸盐与乙醇反应,蒸馏去除过剩的乙醇和副产物乙醚。随后加入30%的NaOH溶液及对甲苯磺酰氯,在水蒸汽蒸馏中去除副产物二乙基苯胺后,再添加硫酸,即可制得目标产品。
- 三氯化磷法:在300℃和9.84MPa的压力下,苯胺、乙醇与三氯化磷反应。将反应混合物通过真空蒸馏分馏,以制备N-乙基苯胺。
类别
有毒物品
毒性分级
中毒
急性毒性
吸入 - 大鼠 LC50: 1130 毫克/立方米/4小时
可燃性危险特性
高温分解,明火条件下可燃;燃烧时会产生有毒氮氧化物和氯化氢烟雾。
储运特性
应存放在通风、低温、干燥的库房中,并与酸类、氧化剂及食品添加剂分开存放。
灭火剂
泡沫、二氧化碳、砂土或干粉均可用于扑灭火灾。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯胺基乙腈 N-phenylglycinonitrile 3009-97-0 C8H8N2 132.165 N-苯基乙醇胺 2-Anilinoethanol 122-98-5 C8H11NO 137.181 N-甲基苯胺 N-methylaniline 100-61-8 C7H9N 107.155 甲基乙基苄基原醇 N-methyl-N-ethylaniline 613-97-8 C9H13N 135.209 1-苯基氮丙啶 1-phenylaziridine 696-18-4 C8H9N 119.166 N-乙酰苯胺 Acetanilid 103-84-4 C8H9NO 135.166 N,N-二乙基苯胺 N,N-diethylaniline 91-66-7 C10H15N 149.236 硫代乙酰苯胺 thioacetanilide 637-53-6 C8H9NS 151.232 1-乙基-1-苯肼 1-ethyl-1-phenylhydrazine 644-21-3 C8H12N2 136.197 N-苯基甲酰胺 Formanilid 103-70-8 C7H7NO 121.139 —— N,N'-diphenyl-ethylidenediamine 5919-63-1 C14H16N2 212.294 2-氯乙酰苯胺 N-chloroacetyl-aniline 587-65-5 C8H8ClNO 169.611 N-烯丙基-N-乙基苯胺 N-allyl-N-ethylaniline 16078-91-4 C11H15N 161.247 —— N-ethyl-N-(prop-2-yn-1-yl)aniline 18158-72-0 C11H13N 159.231 N-乙基甲酰苯胺 N-ethylformanilide 5461-49-4 C9H11NO 149.192 对氯乙酰苯胺 4-Chloroacetanilide 539-03-7 C8H8ClNO 169.611 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲基乙基苄基原醇 N-methyl-N-ethylaniline 613-97-8 C9H13N 135.209 N-乙酰苯胺 Acetanilid 103-84-4 C8H9NO 135.166 —— N-ethyl-1,3-benzenediamine 50617-74-8 C8H12N2 136.197 N,N-二乙基苯胺 N,N-diethylaniline 91-66-7 C10H15N 149.236 —— N-ethyl-4-iodoaniline 68254-65-9 C8H10IN 247.079 N-乙基对甲苯胺 N-ethyl-p-tolylamine 622-57-1 C9H13N 135.209 —— N-sec-butyl-aniline 881008-34-0 C10H15N 149.236 —— N-(1-methylallyl)aniline 70030-17-0 C10H13N 147.22 4-溴-N-乙基苯胺 4-bromo-N-ethylaniline 68254-64-8 C8H10BrN 200.078 P-氨基苯乙醚 (4-hydroxyphenyl)ethylamine 659-34-7 C8H11NO 137.181 乙基氯苯胺 N-ethyl-4-chloro-aniline 13519-75-0 C8H10ClN 155.627 —— 4-nitroso-N-ethylaniline 19788-30-8 C8H10N2O 150.18 —— N-Chloro-N-ethylaniline —— C8H10ClN 155.627 N,N-二甲基苯胺 N,N-dimethyl-aniline 86362-18-7 C8H11N 121.182 1-乙基-1-苯肼 1-ethyl-1-phenylhydrazine 644-21-3 C8H12N2 136.197 N-苯基甲酰胺 Formanilid 103-70-8 C7H7NO 121.139 —— N-(1-methyl-3-butenyl)aniline —— C11H15N 161.247 2-(乙基(苯基)氨基)乙腈 2-[ethyl(phenyl)amino]acetonitrile 16728-93-1 C10H12N2 160.219 N-乙基-N-异丙苯胺 N-ethyl-N-isopropylaniline 54813-77-3 C11H17N 163.263 1,2-环已二酮二肟 N-(2-Aminoethyl)-N-ethylaniline 23730-69-0 C10H16N2 164.25 —— N-ethyl-N-(2-bromo-ethyl)-aniline 827-50-9 C10H14BrN 228.132 3-乙基氨基苯酚 3-(ethylamino)phenol 621-31-8 C8H11NO 137.181 N-烯丙基-N-乙基苯胺 N-allyl-N-ethylaniline 16078-91-4 C11H15N 161.247 N-乙基-N-丙基苯胺 N-Propyl-N-ethylaniline 54813-78-4 C11H17N 163.263 N-乙基-N-氯乙基苯胺 N-ethyl-N-(2-chloroethyl)aniline 92-49-9 C10H14ClN 183.681 N-乙基-N-羟乙基苯胺 2-( N-ethylanilino)ethanol 92-50-2 C10H15NO 165.235 —— N-ethyl-N-(prop-2-yn-1-yl)aniline 18158-72-0 C11H13N 159.231 N-乙基甲酰苯胺 N-ethylformanilide 5461-49-4 C9H11NO 149.192 4-(乙基氨基)苯甲醛(9ci) 4-(ethylamino)benzaldehyde 79865-89-7 C9H11NO 149.192 —— N-Aethylanilinoacetylen 38488-69-6 C10H11N 145.204 1-苯基吡咯烷 N-phenylpyrrolidine 4096-21-3 C10H13N 147.22 N-乙基-邻甲苯胺 N-ethyl-2-methylaniline 94-68-8 C9H13N 135.209 鄰乙胺苯酚 2-ethylaminophenol 614-70-0 C8H11NO 137.181 2-氯-N-乙基苯胺 2-chloro-N-ethylaniline 13519-74-9 C8H10ClN 155.627 N-乙基-4-苯基偶氮苯胺 N-ethyl-4-aminoazobenzene 2058-67-5 C14H15N3 225.293 —— N-ethyl-N'-methyl-N-phenyl-ethylenediamine 55080-47-2 C11H18N2 178.277 苯胺基乙醛二乙基缩醛 N-(2,2-diethoxyethyl)aniline 22758-34-5 C12H19NO2 209.288 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:N-乙基苯胺 在 cobalt(II) chloride 作用下, 生成 N,N-dimethyl-4-ethylaniline参考文献:名称:Davies; Hulbert, Journal of the Society of Chemical Industry, 1938, vol. 57, p. 349摘要:DOI:
-
作为产物:参考文献:名称:使用NaBH 4 / I 2系统还原酰胺,腈,羧酸酯,酸和烯烃加氢硼化的简便方法摘要:酰胺与NaBH 4 -I 2体系在THF中的反应得到相应的胺,产率为70-76%。腈还原后,相应的胺的产率为70-75%。的I 2 /加入NaBH 4系统是在烯烃的hydrocarboration有用和在78-92%的收率后H的所获得的相应的醇2 ö 2 / OH -氧化。该试剂体系还可用于将羧酸酯和酸以60-90%的产率还原为相应的醇。DOI:10.1016/s0040-4020(01)81236-9
-
作为试剂:描述:苯戊酮 在 lithium aluminium tetrahydride 、 N-甲基麻黄碱 、 N-乙基苯胺 作用下, 以 乙醚 为溶剂, 生成 (-)-(S)-1-苯基戊-1-醇 、 (1R)-1-苯基戊-1-醇参考文献:名称:用 (-)-N-甲基麻黄碱和 N-乙苯胺部分分解的氢化铝锂不对称还原简单的非手性酮摘要:发现标题不对称还原以高光学(最大 90% ee)和化学(最大 100%)产率得到相应的旋光醇。DOI:10.1246/cl.1980.981
文献信息
-
[EN] NOVEL COMPOUNDS, THEIR PREPARATION AND USE<br/>[FR] NOUVEAUX COMPOSES, LEUR PREPARATION ET LEUR UTILISATION申请人:NOVO NORDISK AS公开号:WO2005105736A1公开(公告)日:2005-11-10Novel compounds of the general formula (I), the use of these compounds as phar- maceutical compositions, pharmaceutical compositions comprising the compounds and methods of treatment employing these compounds and compositions. The present compounds may be useful in the treatment and/or prevention of conditions mediated by Peroxisome Proliferator-Activated Receptors (PPAR), in particular the PPARδ suptype.
-
The titanocene-catalyzed reduction of acetamides to tertiary amines by PhMeSiH<sub>2</sub>作者:Kumaravel Selvakumar、Kesamreddy Rangareddy、John F HarrodDOI:10.1139/v04-063日期:2004.8.1
A variety of acetamide derivatives are reduced in excellent yields to tertiary amines by PhMeSiH2 in the presence of Cp2TiX2 (X = F or Me) catalysts. The reactions are very clean at 80 °C. At room temperature a secondary reaction, hydrogenolysis of the C(O)N bond, intervenes and reduces the chemoselectivity. Nevertheless, this chemistry provides a simple methodology for the amide/alkylamine transformation using inexpensive, commercially available reagents.Key words: amides, reduction, secondary amides, methylphenylsilane, titanocene, catalysis.
-
Homogeneous Catalytic Hydrogenation of Amides to Amines作者:Jacorien Coetzee、Deborah L. Dodds、Jürgen Klankermayer、Sandra Brosinski、Walter Leitner、Alexandra M. Z. Slawin、David J. Cole-HamiltonDOI:10.1002/chem.201204270日期:2013.8.12Hydrogenation of amides in the presence of [Ru(acac)3] (acacH=2,4‐pentanedione), triphos [1,1,1‐tris‐ (diphenylphosphinomethyl)ethane] and methanesulfonic acid (MSA) produces secondary and tertiary amines with selectivities as high as 93 % provided that there is at least one aromatic ring on N. The system is also active for the synthesis of primary amines. In an attempt to probe the role of MSA and在[Ru(acac)3 ](acacH = 2,4-戊二酮),三[[1,1,1-三(二苯基膦甲基)乙烷]]和甲磺酸(MSA)的存在下进行酰胺加氢生成仲胺和叔胺如果在N上至少有一个芳环,则其选择性高达93%。该系统对伯胺的合成也具有活性。为了探索MSA的作用和反应机理,已经从[Ru(acac)3 ],三醇和MSA或[RuX(OAc)(triphos)]的反应中制备了一系列甲磺酸钠络合物。 (X = H或OAc)或[RuH 2(CO)(triphos )]与MSA。晶体学表征复合物包括:[茹(OAC-κ 1 O)2(H 2O)(triphos)],[Ru(OAc‐κ 2 O,O')(CH 3 SO 3 ‐κ 1 O)(triphos )],[Ru(CH 3 SO 3‐ κ 1 O)2(H 2 O)(三膦)]和[孺2(μ-CH 3 SO 3)3(三磷酸)2 ] [CH 3 SO 3 ],而其他复合物,例如[茹(OAC-κ
-
A General Proline‐Catalyzed Synthesis of 4,5‐Disubstituted <i>N</i> ‐Sulfonyl‐1,2,3‐Triazoles from 1,3‐Dicarbonyl Compounds and Sulfonyl Azide作者:Shanmugam Rajasekar、Pazhamalai AnbarasanDOI:10.1002/asia.201901015日期:2019.12.13An efficient proline-catalyzed synthesis of 4,5-disubstituted-N-sulfonyl-1,2,3-triazoles has been accomplished from 1,3-dicarbonyl compounds and sulfonyl azides. The developed reaction is suitable for various symmetrical and unsymmetrical 1,3-dicarbonyl compounds, tolerates various functional groups and affords 4,5-disubstituted-N-sulfonyl-1,2,3-triazoles in good yield with excellent regioselectivity
-
Efficient Synthesis of N-Sulfonylacetamidines from Propiolic Acid by Copper-Catalyzed Three-Component Coupling Reactions作者:Chunxiang Kuang、Qing Yang、Mei Xu、Zhuo WangDOI:10.1055/s-0030-1258587日期:2010.10Using propiolic acid as one of the reacting components, a copper-catalyzed three-component reaction of sulfonyl azide and amines affords N-sulfonylacetamidines via a decarboxylation process in high yields under mild conditions. This one-pot method is efficient, general, and versatile.
表征谱图
-
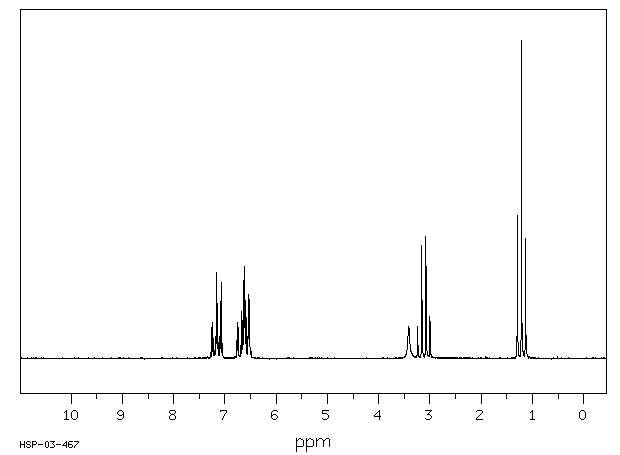
氢谱1HNMR
-
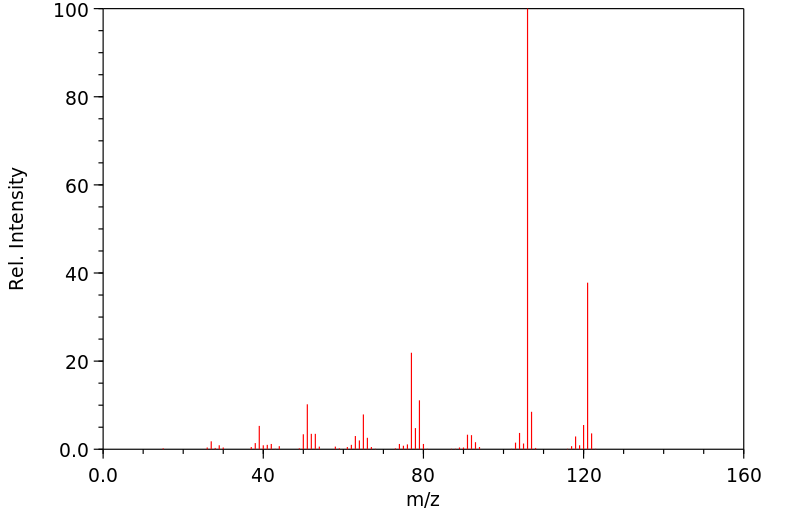
质谱MS
-
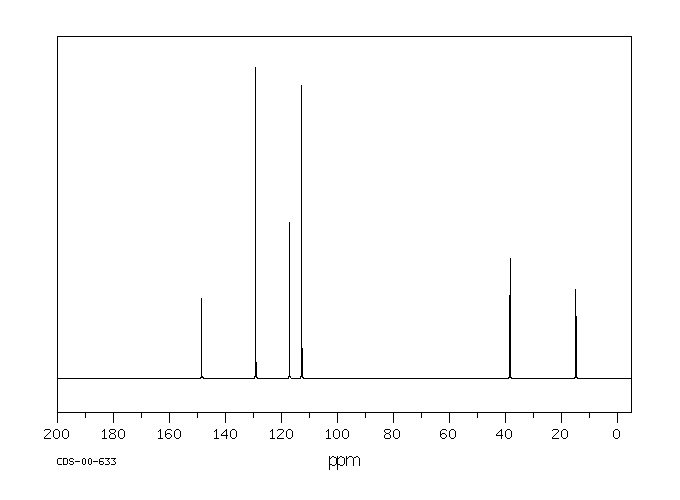
碳谱13CNMR
-
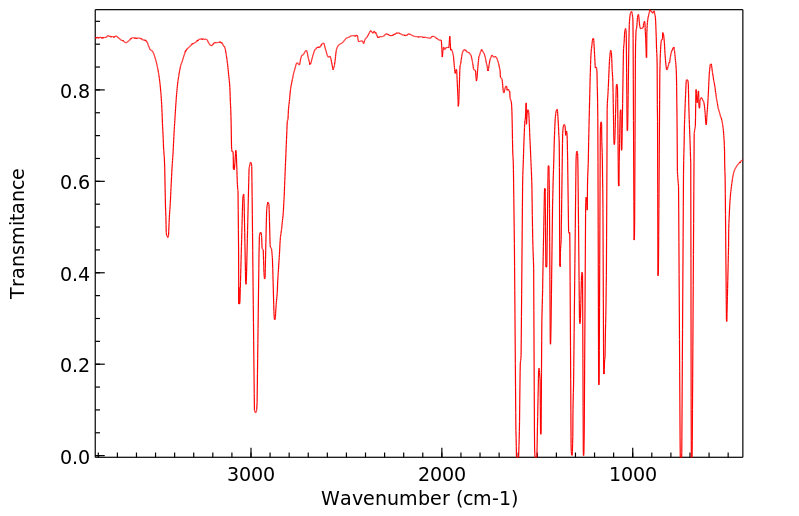
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息