1-苯基吡咯烷 | 4096-21-3
中文名称
1-苯基吡咯烷
中文别名
1-苯基吡咯啉;N-苯基四氢吡咯
英文名称
N-phenylpyrrolidine
英文别名
1-phenylpyrrolidine;N-phenylpyrolidine;N-phenyltetrahydropyrrole;NPP
CAS
4096-21-3
化学式
C10H13N
mdl
MFCD00015897
分子量
147.22
InChiKey
VDQQJMHXZCMNMU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:11°C
-
沸点:133-134°C 19mm
-
密度:1.0180
-
闪点:133-134°C/19mm
-
稳定性/保质期:
在常温常压下,这是一种稳定的油状液体。它的沸点为119-120℃(1.6kPa)和81℃(0.06kPa)。相对密度为1.0260(25/4℃),折光率为1.5813,并且可以溶于乙醚。
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:11
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S28,S36/37
-
危险类别码:R21/22
-
危险品运输编号:2810
-
海关编码:2933990090
-
包装等级:III
-
危险性防范说明:P280
-
危险性描述:H302,H312
-
储存条件:请将产品存放在避光、通风干燥的地方,并密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-Phenylpyrrolidine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H311: Toxic in contact with skin
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P361: Remove/Take off immediately all contaminated clothing
P312: Call a POISON CENTER or doctor/physician if you feel unwell
P405: Store locked up
Section 3. Composition/information on ingredients.
Ingredient name: 1-Phenylpyrrolidine
CAS number: 4096-21-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C10H13N
Molecular weight: 147.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-Phenylpyrrolidine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H311: Toxic in contact with skin
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P361: Remove/Take off immediately all contaminated clothing
P312: Call a POISON CENTER or doctor/physician if you feel unwell
P405: Store locked up
Section 3. Composition/information on ingredients.
Ingredient name: 1-Phenylpyrrolidine
CAS number: 4096-21-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C10H13N
Molecular weight: 147.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(4-碘苯基)吡咯烷 1-(4-iodophenyl)pyrrolidine 87350-76-3 C10H12IN 273.116 —— 1-(4-fluorophenyl)pyrrolidine 4280-34-6 C10H12FN 165.21 1-(3-氟苯基)吡咯烷 1-(3-fluorophenyl)pyrrolidine 139909-17-4 C10H12FN 165.21 —— N-4-Chlorbutylanilin 42330-98-3 C10H14ClN 183.681 —— 4-(phenylamino)butan-1-ol 6517-80-2 C10H15NO 165.235 N,N-双(2-溴乙基)苯胺 N,N-bis(2-bromoethyl)aniline 2045-19-4 C10H13Br2N 307.028 N,N-二烯丙苯胺 N,N-diallylaniline 6247-00-3 C12H15N 173.258 1-苯基-2-吡咯烷酮 1-phenylpyrrolidin-2-one 4641-57-0 C10H11NO 161.203 1-(2-氟苯基)吡咯烷 1-(2-fluorophenyl)pyrrolidine 758691-88-2 C10H12FN 165.21 —— N-(3-butenyl)aniline 29369-71-9 C10H13N 147.22 N-苯丁二醯亞胺 N-phenylmaleimide 83-25-0 C10H9NO2 175.187 N-乙基苯胺 N-ethyl-N-phenylamine 103-69-5 C8H11N 121.182 —— methyl 4-(phenylamino)butanoate 91246-76-3 C11H15NO2 193.246 N,N-二甲基苯胺 N,N-dimethyl-aniline 86362-18-7 C8H11N 121.182 N-异丙基苯胺 N-Isopropylaniline 768-52-5 C9H13N 135.209 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(4-碘苯基)吡咯烷 1-(4-iodophenyl)pyrrolidine 87350-76-3 C10H12IN 273.116 N-对溴苯吡咯烷 1-(4-bromo-phenyl)-pyrrolidine 22090-26-2 C10H12BrN 226.116 4-吡咯烷苯硫酚 4-Pyrrolidinothiophenol 106365-88-2 C10H13NS 179.286 —— 1-(4-nitrosophenyl)pyrrolidine 52695-15-5 C10H12N2O 176.218 4-(1-吡咯啉基)苯甲醛 4-(pyrrolidin-1-yl)benzaldehyde 51980-54-2 C11H13NO 175.23 —— 1-(4-isothiocyanatophenyl)pyrrolidine 51317-64-7 C11H12N2S 204.296 —— N-butyl-N-phenylformamide 35082-00-9 C11H15NO 177.246 —— 4,4'-di(pyrrolidin-1-yl)-1,1'-biphenyl 959276-94-9 C20H24N2 292.424 —— 1-([1,1'-biphenyl]-4-yl)pyrrolidine 265991-28-4 C16H17N 223.318 1-[4-[(4-吡咯烷-1-基苯基)甲基]苯基]吡咯烷 Bis(4-pyrrolidinophenyl)methane 53926-61-7 C21H26N2 306.451 1-(4-硝基苯基)吡咯烷 1-(4-nitro-phenyl)-pyrrolidine 10220-22-1 C10H12N2O2 192.217 1-苯基-2-吡咯烷酮 1-phenylpyrrolidin-2-one 4641-57-0 C10H11NO 161.203 1-(2-溴苯基)吡咯烷 N-(2-bromophenyl)-pyrrolidine 87698-81-5 C10H12BrN 226.116 1-苯基吡咯烷-2-硫酮 N-Phenyl-γ-butyrothiolactam 58982-96-0 C10H11NS 177.27 4’-(1-吡咯烷基)苯乙酮 N-(4-acetylphenyl)pyrrolidine 21557-09-5 C12H15NO 189.257 4-吡咯烷-1-基苯甲酰基氯化物 4-(pyrrolidin-1-yl)benzoyl chloride 679809-11-1 C11H12ClNO 209.675 4-(1-吡咯烷基)苯甲酸 4-(pyrrolidin-1-yl)benzoic acid 22090-27-3 C11H13NO2 191.23 1-[4-[苯基-(4-吡咯烷-1-基苯基)甲基]苯基]吡咯烷 4,4'-dipyrrolidinotriphenylmethane 87872-24-0 C27H30N2 382.549 —— 4-(4-(pyrrolidin-1-yl)phenyl)butan-2-one —— C14H19NO 217.311 —— 1-phenylpyrrolidine-2-carbonitrile —— C11H12N2 172.23 —— (E)-4-(4-(pyrrolidin-1-yl)styryl)bromobenzene 1350615-96-1 C18H18BrN 328.252 —— (E)-4-(4-(pyrrolidin-1-yl)styryl)benzaldehyde 1350616-02-2 C19H19NO 277.366 —— (R)-3-(4-Pyrrolidin-1-yl-phenyl)-butan-1-ol 637762-39-1 C14H21NO 219.327 —— (R)-3-(4-pyrrolidin-1-yl-phenyl)-butyraldehyde 447461-88-3 C14H19NO 217.311 —— 1,1-Bis(4-pyrrolidinylphenyl)ethane 105443-13-8 C22H28N2 320.5 4-(吡咯烷-1-基)苯-1-磺酰氯 4-(1-pyrrolidyl)benzenesulfonyl chloride 125393-18-2 C10H12ClNO2S 245.73 4-吡咯烷-1-基苯磺酸 4-(1-pyrrolidyl)benzenesulfonic acid 853789-34-1 C10H13NO3S 227.284 —— (4-methoxyphenyl)-(4-pyrrolidin-1-ylphenyl)diazene 89505-20-4 C17H19N3O 281.357 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Reppe et al., Justus Liebigs Annalen der Chemie, 1955, vol. 596, p. 1,145摘要:DOI:
-
作为产物:描述:N-苯基-4-甲氧基苯甲酰胺 在 三(乙酰丙酮酸)钌(III) 、 ytterbium(III) triflate hydrate 、 氢气 、 1,1,1-三(二苯基膦甲基)乙烷 作用下, 以 四氢呋喃 为溶剂, 150.0 ℃ 、500.01 kPa 条件下, 反应 30.0h, 生成 1-苯基吡咯烷参考文献:名称:实现仲酰胺和叔酰胺向胺的一般钌催化氢化†摘要:使用 [Ru(acac) 3 ]、1,1,1-三(二苯基膦甲基)乙烷 (Triphos) 和 Yb组合生成的原位催化剂,在温和条件下将多种仲酰胺和叔酰胺氢化成相应的胺(OTf)3。与之前的报道相比,金属三氟甲磺酸盐的存在可以减轻反应条件,从而提高所需胺的产率和选择性。两次放大实验的优异分离产率证实了反应方案的可行性。对照实验表明,在酰胺羰基最初还原后,反应通过醇与中间体半缩醛胺塌陷产生的胺进行还原胺化来进行。DOI:10.1039/c5sc04671h
-
作为试剂:参考文献:名称:JP2005/350416摘要:公开号:
文献信息
-
Dye–polyoxometalate coordination polymer as a photo-driven electron pump for photocatalytic radical coupling reactions作者:Zheng Ming、Tiexin Zhang、Wenming Tian、Jianing Li、Zhenhui Liu、Renhai Liu、Zhongmin Liu、Chunying DuanDOI:10.1039/d1cc04209b日期:——To alleviate diffusion-limited photoinduced electron transfer (PET) in solution, a triphenylamine-derived dye and a Keggin polyoxometalate-type electron relay were coupled into a coordination polymer to photoinduce long-lived charge-separation pairs with enough reductive/oxidative potential to pump multiple electrons unidirectionally from external electron donors to acceptors, thus furnishing photocatalytic
-
Borane-Methyl Sulfide Reductive Cyclization of ω-Ester Alkylamides: A Convenient Synthesis of<i>N</i>-Substituted Cyclic Amines作者:Michael C. Venuti、Oswald OrtDOI:10.1055/s-1988-27777日期:——Borane-methyl sulfide (BMS) reduction of variously N-substituted succinamic and glutaramic esters affords the corresponding N-substituted pyrrolidines and piperidines in high yields. The limitations, mainly caused by steric hinderance around the amine nitrogen, and putative intermediates involved in this conversion, as detected by incomplete reaction and/or synthesis followed by BMS reduction, indicate that cyclization and amide reduction successfully compete with ester reduction to afford the N-substituted cyclized amines.
-
The titanocene-catalyzed reduction of acetamides to tertiary amines by PhMeSiH<sub>2</sub>作者:Kumaravel Selvakumar、Kesamreddy Rangareddy、John F HarrodDOI:10.1139/v04-063日期:2004.8.1
A variety of acetamide derivatives are reduced in excellent yields to tertiary amines by PhMeSiH2 in the presence of Cp2TiX2 (X = F or Me) catalysts. The reactions are very clean at 80 °C. At room temperature a secondary reaction, hydrogenolysis of the C(O)N bond, intervenes and reduces the chemoselectivity. Nevertheless, this chemistry provides a simple methodology for the amide/alkylamine transformation using inexpensive, commercially available reagents.Key words: amides, reduction, secondary amides, methylphenylsilane, titanocene, catalysis.
-
Diisobutylaluminum borohydride: An efficient reagent for the reduction of tertiary amides to the corresponding amines under ambient conditions作者:Rachel A. Snelling、Gabriella Amberchan、Angel Resendez、Chris L. Murphy、Lauren Porter、Bakthan SingaramDOI:10.1016/j.tetlet.2017.09.030日期:2017.10A synthetically simple mixed metal hydride, diisobutylaluminum borohydride [(iBu)2AlBH4], is easily generated from a 1:1 mixture of borane-dimethylsulfide (BMS) and diisobutylaluminum hydride (DIBAL). The reduction of tertiary amides using (iBu)2AlBH4 is complete within five minutes under ambient conditions and the product tertiary amines were isolated in 70–99% yields by a simple acid-base extraction
-
Reaction of Diisobutylaluminum Borohydride, a Binary Hydride, with Selected Organic Compounds Containing Representative Functional Groups作者:Gabriella Amberchan、Rachel A. Snelling、Enrique Moya、Madison Landi、Kyle Lutz、Roxanne Gatihi、Bakthan SingaramDOI:10.1021/acs.joc.0c03062日期:2021.5.7from diisobutylaluminum hydride (DIBAL) and borane dimethyl sulfide (BMS) has shown great potential in reducing a variety of organic functional groups. This unique binary hydride, (iBu)2AlBH4, is readily synthesized, versatile, and simple to use. Aldehydes, ketones, esters, and epoxides are reduced very fast to the corresponding alcohols in essentially quantitative yields. This binary hydride can reduce由氢化二异丁基铝(DIBAL)和硼烷二甲基硫醚(BMS)合成的二元氢化物二异丁基硼氢化铝[(i Bu)2 AlBH 4 ]在还原各种有机官能团方面显示出巨大潜力。这种独特的二元氢化物(i Bu)2 AlBH 4易于合成,通用且易于使用。醛,酮,酯和环氧化物以基本定量的收率非常快地还原为相应的醇。该二元氢化物可以在25°C下以有效方式将叔酰胺迅速还原为相应的胺。此外,腈以基本上定量的产率转化为相应的胺。这些反应在环境条件下发生,并在一个小时或更短的时间内完成。还原产物可通过简单的酸碱萃取而分离,无需使用柱色谱法。进一步的研究表明(i Bu)2 AlBH 4如一系列竞争反应所示,它具有成为选择性氢化物供体的潜力。讨论了(i Bu)2 AlBH 4,DIBAL和BMS之间的异同。
表征谱图
-
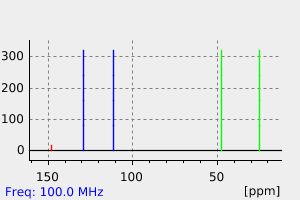
氢谱1HNMR
-
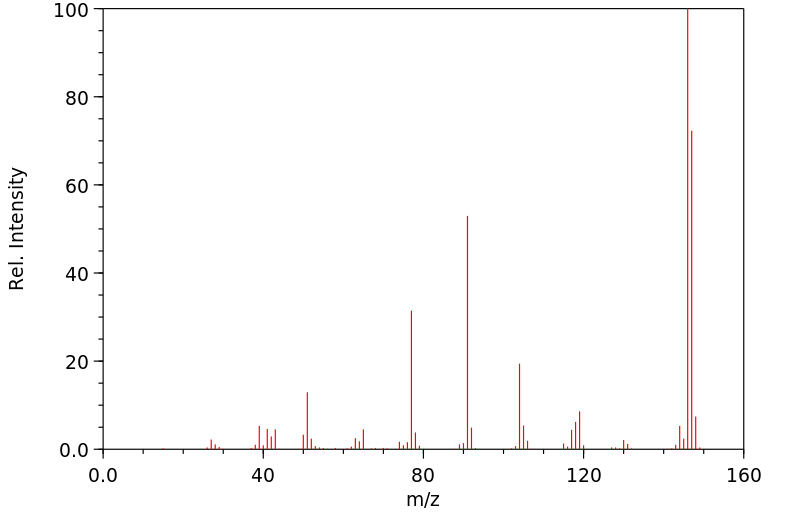
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5R,Z)-3-(羟基((1R,2S,6S,8aS)-1,3,6-三甲基-2-((E)-prop-1-en-1-yl)-1,2,4a,5,6,7,8,8a-八氢萘-1-基)亚甲基)-5-(羟甲基)-1-甲基吡咯烷-2,4-二酮
(2R,2''R)-(-)-2,2''-联吡咯烷
麦角甾-7,22-二烯-3-基亚油酸酯
马来酰亚胺霉素
马来酰亚胺基酰肼盐酸盐
马来酰亚胺基甲基-3-马来酰亚胺基丙酸酯
马来酰亚胺丙酰基-dPEG4-NHS
马来酰亚胺-酰胺-PEG6-琥珀酰亚胺酯
马来酰亚胺-酰胺-PEG6-丙酸
马来酰亚胺-酰胺-PEG24-丙酸
马来酰亚胺-酰胺-PEG12-丙酸
马来酰亚胺-四聚乙二醇-羧酸
马来酰亚胺-四聚乙二醇-丙酸叔丁酯
马来酰亚胺-四聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-六聚乙二醇-羧酸
马来酰亚胺-六聚乙二醇-丙酸叔丁酯
马来酰亚胺-八聚乙二醇-丙酸叔丁酯
马来酰亚胺-二聚乙二醇-丙酸叔丁酯
马来酰亚胺-三(乙烯乙二醇)-丙酸
马来酰亚胺-一聚乙二醇-羧酸
马来酰亚胺-一聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-PEG3-羟基
马来酰亚胺-PEG2-胺三氟醋酸盐
马来酰亚胺-PEG2-琥珀酰亚胺酯
马来酰亚胺
频哪醇硼酸酯
顺式草酸双(-3,8-二氮杂双环[4.2.0]辛烷-8-羧酸叔丁酯)
顺式4-甲基吡咯烷酮-3-醇盐酸盐
顺式4-氟吡咯烷酮-3-醇盐酸盐
顺式3,4-二羟基吡咯烷盐酸盐
顺式3,4-二氨基吡咯烷-1-羧酸叔丁酯
顺式-二甲基 1-苄基吡咯烷-3,4-二羧酸
顺式-N-[2-(2,6-二甲基-1-哌啶基)乙基]-2-氧代-4-苯基-1-吡咯烷乙酰胺
顺式-N-Boc-吡咯烷-3,4-二羧酸
顺式-5-苄基-2-叔丁氧羰基六氢吡咯并[3,4-c]吡咯
顺式-5-甲基-1H-六氢吡咯并[3,4-b]吡咯二盐酸盐
顺式-5-氧代六氢环戊二烯并[c]吡咯-2(1H)-羧酸叔丁酯
顺式-5-乙氧羰基-1H-六氢吡咯并[3,4-B]吡咯盐酸盐
顺式-5-(碘甲基)-4-苯基-2-吡咯烷酮
顺式-5-(碘甲基)-4-甲基-2-吡咯烷酮
顺式-4-氧代-六氢-吡咯并[3,4-C]吡咯-2-甲酸叔丁酯
顺式-3-氟-4-羟基吡咯烷-1-羧酸叔丁酯
顺式-3-氟-4-甲基吡咯烷盐酸盐
顺式-2-甲基六氢吡咯并[3,4-c]吡咯
顺式-2,5-二甲基吡咯烷
顺式-1-苄基-3,4-吡咯烷二甲酸二乙酯
顺式-1-甲基六氢吡咯并[3,4-b]吡咯
顺式-(9CI)-3,4-二乙烯-1-(三氟乙酰基)-吡咯烷
顺-八氢环戊[c]吡咯-5-酮盐酸盐
非星匹宁