3-甲基-4-戊醇 | 51174-44-8
物质功能分类
中文名称
3-甲基-4-戊醇
中文别名
——
英文名称
3-methyl-4-penten-1-ol
英文别名
3-methylpent-4-en-1-ol;3-methyl-4-pentenol;3-methylpent-1-en-5-ol;4-Penten-1-ol, 3-methyl-
CAS
51174-44-8
化学式
C6H12O
mdl
MFCD00020621
分子量
100.161
InChiKey
VTCQTYOGWYLVES-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:22.55°C (estimate)
-
沸点:152.63°C (estimate)
-
密度:0.8489 (estimate)
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-甲基-4-戊烯酸 3-methyl-pent-4-enoic acid 1879-03-4 C6H10O2 114.144
反应信息
-
作为反应物:描述:参考文献:名称:Ring Closure Reactions of Substituted 4-Pentenyl-1-oxy Radicals. The Stereoselective Synthesis of Functionalized Disubstituted Tetrahydrofurans摘要:N-(Alkyloxy)pyridine-2( VT)-thiones 3 and benzenesulfenic acid O-esters 5 have been synthesized from substituted 4-pentenols 1 or the derived tosylates. Compounds 3 and 5 are efficient sources of free alkoxy radicals 6 which undergo synthetically useful fast ring closure reactions 6 --> 8 [k(exo) = (2 +/- 1) x 10(8) s(-1) to (6 +/- 2) x 10(9) s(-1) (T = 30 +/- 0.2 degrees C)]. Tetrahydrofurfuryl radicals 8 can be trapped with, e.g., hydrogen or chlorine atom donors to afford either trans- or cis-disubstituted tetrahydrofurans 10 or 12 depending on the substitution pattern of the 4-pentenyloxy radical. Substituted tetrahydropyrans 11 or 13 are formed in the minor 6-endo-trig cyclization. According to the data of competition kinetics, the observed stereoselectivities in free alkoxy radical cyclizations arise from steric interactions between the substituents in the transition state of the ring closure reactions. Alkyl substituents cause small differences in the measured relative rate constants of B-exo cyclizations which are reminiscent of the data obtained from the rearrangements of alkyl-substituted 5-hexenyl radicals. Likewise, a stereochemical model for oxygen radical cyclization is proposed where the pentenyloxy chain adopts a six-membered, chairlike transition state with the alkyl substituents preferentially situated in the pseudoequatorial positions leading to 2,5-trans-, 2,4-cis-, and 2,3-trans-substituted tetrahydrofurfuryl radicals 8 as the major intermediates.DOI:10.1021/jo00126a021
-
作为产物:描述:参考文献:名称:Synthesis of 4,4′-Disubstituted Azepines via Ring-Closing Metathesis Reaction and Asymmetric Arylation of Lactones摘要:标题化合物的合成通过一系列独特的反应实现,包括使用第二代Grubbs催化剂对ω-二烯进行闭环复分解反应。手性二烯前体可以从相应的α,α′-二取代内酯衍生物中获得,具有外消旋或光学富集形式。DOI:10.1055/s-2006-926433
文献信息
-
[EN] COMPOUNDS THAT INHIBIT MCL-1 PROTEIN<br/>[FR] COMPOSÉS INHIBANT LA PROTÉINE MCL-1申请人:AMGEN INC公开号:WO2017147410A1公开(公告)日:2017-08-31Provided herein are myeloid cell leukemia 1 protein (Mcl-1) inhibitors, methods of their preparation, related pharmaceutical compositions, and methods of using the same. For example, provided herein are compounds of Formula I, and pharmaceutically acceptable salts thereof and pharmaceutical compositions containing the compounds. The compounds and compositions provided herein may be used, for example, in the treatment of diseases or conditions, such as cancer.本文提供了髓样细胞白血病1蛋白(Mcl-1)抑制剂,其制备方法,相关的药物组合物,以及使用这些物质的方法。例如,本文提供了化合物I的公式,及其药用盐和含有这些化合物的药物组合物。本文提供的化合物和组合物可以用于治疗癌症等疾病或症状。
-
[EN] AZABICYCLIC(THIO)AMIDES AS FUNGICIDAL COMPOUNDS<br/>[FR] (THIO)AMIDES AZABICYCLIQUES EN TANT QUE COMPOSÉS FONGICIDES申请人:BAYER AG公开号:WO2021233861A1公开(公告)日:2021-11-25The present invention relates to azabicyclic (thio)amide compounds and the uses thereof for controlling phytopathogenic microorganisms such as phytopathogenic fungi. It also relates to processes and intermediates for preparing these compounds
-
Intramolecular Pyridinium Oxide Cycloadditions: Systematic Study of Substitution, Diastereoselectivity, and Regioselectivity作者:Yi Lu、Patrick N. Dey、Christopher M. BeaudryDOI:10.1002/chem.202100115日期:2021.2.24Intramolecular pyridinium oxide cycloadditions form complex polycyclic nitrogenous architectures. The diastereoselectivity and regioselectivity of pyridinium oxide cycloadditions was systematically investigated for the first time using complex substrates. Predictably high levels of diastereoselectivity and regioselectivity are observed, which can be attributed to minimization of steric (syn‐pentane)
-
Pd(II)-Catalyzed [4 + 2] Heterocyclization Sequence for Polyheterocycle Generation作者:Elizabeth L. Glaisyer、Michael S. Watt、Kevin I. Booker-MilburnDOI:10.1021/acs.orglett.8b02543日期:2018.9.21A new Pd(II)-catalyzed cascade sequence for the formation of polyheterocycles, from simple starting materials, is reported. The sequence is applicable to both indole and pyrrole substrates, and a range of substituents are tolerated. The reaction is thought to proceed by a Pd(II)-catalyzed C–H activated Heck reaction followed by a second Pd(II)-catalyzed aza-Wacker reaction with two Cu(II)-mediated
-
[DE] VERFAHREN ZUR HERSTELLUNG VON 3-PHENYL(THIO)URACILEN UND - DITHIOURACILEN<br/>[EN] METHOD FOR THE PRODUCTION OF 3-PHENYL(THIO)URACILS AND DITHIOURACILS<br/>[FR] PROCEDE DE PRODUCTION DE 3-PHENYL(THIO)URACILES ET DE 3-PHENYL-DITHIO-URACILES申请人:BASF AG公开号:WO2006010474A1公开(公告)日:2006-02-02Die vorliegende Erfindung betrifft ein Verfahren zur Herstellung von 3-Phenyl(thio)uracilen und -dithiouracilen der Formel I, worin die Variablen die in der Beschreibung genannten Bedeutungen haben, dadurch gekennzeichnet, dass Carbamate der Formel II, wobei die Variablen X1, X3, Ar und A die zuvor genannten Bedeutungen haben und L1für eine nucleophil verdrängbare Abgangsgruppe steht, mit Enaminen der Formel III, wobei die Variablen X2, R1, R2 und R3 die zuvor genannten Bedeutungen haben und L2 für eine nucleophil verdrängbare Abgangsgruppe steht, umgesetzt werden, sowie Zwischenprodukte zu ihrer Herstellung.
表征谱图
-
氢谱1HNMR
-
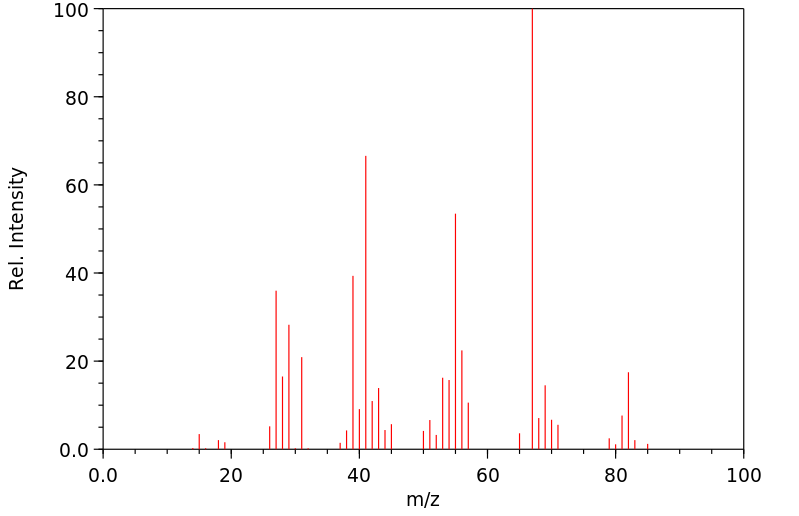
质谱MS
-
碳谱13CNMR
-
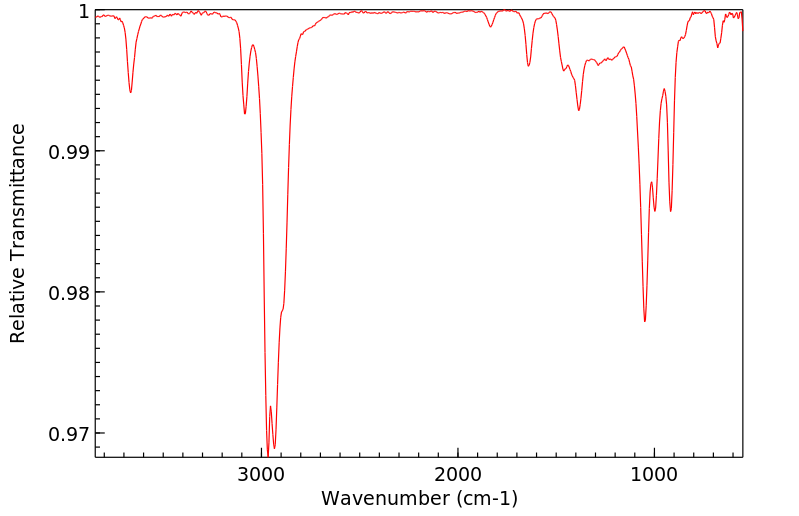
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷