2-氯-4-硝基苯腈 | 28163-00-0
中文名称
2-氯-4-硝基苯腈
中文别名
2-氯-4-硝基苯甲腈;2-氯-4-硝基苄腈
英文名称
2-chloro-4-nitrobenzonitrile
英文别名
2-Chlor-4-nitro-benzonitril;4-nitro-2-chlorobenzonitrile
CAS
28163-00-0
化学式
C7H3ClN2O2
mdl
——
分子量
182.566
InChiKey
ZIGQFRQZYVQQDR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:83-84 °C
-
沸点:337.0±27.0 °C(Predicted)
-
密度:1.6133 (rough estimate)
-
溶解度:溶于甲醇
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,目前没有发现任何已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:69.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S22,S26,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2926909090
-
危险品运输编号:3276
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将贮藏器密封保存,并存放在阴凉干燥处。同时,确保工作环境有足够的通风或排气设施。
SDS
| Name: | 2-Chloro-4-Nitrobenzonitrile Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 28163-00-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 28163-00-0 | 2-Chloro-4-Nitrobenzonitrile | ca. 100 | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Metabolism may release cyanide, which may result in headache, dizziness, weakness, collapse, unconsciousness and possible death.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema. May be metabolized to cyanide which in turns act by inhibiting cytochrome oxidase impairing cellular respiration.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use only in a chemical fume hood. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 28163-00-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 78 - 80 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H3ClN2O2
Molecular Weight: 182.4862
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents, strong bases, strong acids.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 28163-00-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Chloro-4-Nitrobenzonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 28163-00-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 28163-00-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 28163-00-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:用于制作染料中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 alpha,2-二氯-4-硝基甲苯 2-chloro-4-nitrobenzyl chloride 50274-95-8 C7H5Cl2NO2 206.028 2-氯-4-硝基苯甲醛 2-chloro-4-nitrobenzaldehyde 5568-33-2 C7H4ClNO3 185.567 (2-氯-4硝基苄醇)甲醇 (2-chloro-4-nitrophenyl)methanol 52301-88-9 C7H6ClNO3 187.583 1-溴甲基-2-氯-4-硝基苯 1-(bromomethyl)-2-chloro-4-nitrobenzene 42533-63-1 C7H5BrClNO2 250.479 阿克洛胺 Novastat 3011-89-0 C7H5ClN2O3 200.581 对硝基苯甲腈 4-nitrobenzonitrile 619-72-7 C7H4N2O2 148.121 2-氯-4-硝基苯胺 2-Chloro-4-nitroaniline 121-87-9 C6H5ClN2O2 172.571 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氯-4-氨基苯腈 4-amino-2-chlorobenzonitrile 20925-27-3 C7H5ClN2 152.583 2-氯-4-硝基苯甲酸 2-chloro-4-nitrobenzoic acid 99-60-5 C7H4ClNO4 201.566 —— 2-chloro-N'-hydroxy-4-nitrobenzamidine 96898-76-9 C7H6ClN3O3 215.596
反应信息
-
作为反应物:描述:2-氯-4-硝基苯腈 在 盐酸 、 aluminum (III) chloride 、 氯化亚砜 、 palladium on activated charcoal 、 氢气 、 sodium ethanolate 、 溶剂黄146 、 2,3-二氯-5,6-二氰基-1,4-苯醌 、 zinc(II) oxide 、 sodium hydroxide 作用下, 以 甲醇 、 四氯乙烯 、 乙醇 、 乙酸乙酯 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 49.0h, 生成 乐伐替尼参考文献:名称:一种乐伐替尼的制备方法摘要:本发明公开了一种乐伐替尼的制备方法,4‑硝基‑2‑氯苯甲腈为起始原料,在分子中引入硝基吸电子,使得苯环的电子云密度大大降低,有利于亲核取代反应,并大大降低反应的温度;氨基保护,采用ZnO作为路易斯酸,使得该步反应绿色化;通过付克酰基化,脱保护,分子内烷基化关环,相对于传统的高温反应,本申请合成路线新颖,反应温度低,条件温和、绿色,无危险步骤,适合工业化生产。公开号:CN110981800A
-
作为产物:描述:参考文献:名称:Anthelmintic 1-(substituted phenyl)-3-alkanimidoyl ureas摘要:本发明的化合物是具有驱虫活性的新型咪唑脲和具有抗生育活性的咪唑硫脲。它们是通过适当的脲类化合物与适当的异氰酸酯或异硫氰酸酯反应制备而成。公开号:US03984467A1
文献信息
-
Substituted phenyl farnesyltransferase inhibitors申请人:——公开号:US20020019527A1公开(公告)日:2002-02-14Compounds of formula (I) 1 or pharmaceutically acceptable salts thereof, inhibit farnesyltransferase. Methods for making the compounds, pharmaceutical compositions containing the compounds, and methods of treatment using the compounds are disclosed.式(I)的化合物或其药学上可接受的盐,抑制法尼基转移酶。公开了制备这些化合物的方法,含有这些化合物的药物组合物,以及使用这些化合物进行治疗的方法。
-
Direct oxidative conversion of benzylhalides, -amines, -alcohols, and arylaldehydes to nitriles with trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane activated by NH4Br作者:Davood Azarifar、Zohreh NajminejadDOI:10.1007/s13738-014-0461-3日期:2015.1A simple and efficient oxidative conversion of benzyl derivatives of halides, amines, alcohols, and aldehydes into corresponding nitriles is described using trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane in the presence of NH4Br. The reactions proceeded smoothly at room temperature to afford the products in high-to-excellent yields.报道了一种简单高效的氧化转化方法,使用NH4Br存在下的反式-3,5-二氢过氧-3,5-二甲基-1,2-二氧杂环戊烷,将卤化苄、胺基苄、醇基苄和醛基苄转化为相应的腈。反应在室温下顺利进行,产率从高到优异。
-
[EN] TRIAZOLE COMPOUNDS AS ANTIVIRALS<br/>[FR] COMPOSÉS TRIAZOLES EN TANT QU'ANTIVIRAUX申请人:HOFFMANN LA ROCHE公开号:WO2014006066A1公开(公告)日:2014-01-09The present invention discloses compounds of Formula I: wherein the variables in Formula I are defined as described herein. Also disclosed are pharmaceutical compositions containing such compounds and methods for using the compounds of Formula I in the prevention or treatment of HCV infection.本发明公开了公式I的化合物:其中公式I中的变量定义如本文所述。还公开了包含此类化合物的药物组合物以及使用公式I化合物预防或治疗HCV感染的方法。
-
First palladium-catalyzed denitrated coupling of nitroarenes with sulfinates作者:Heng Tian、Aijuan Cao、Lijun Qiao、Ajuan Yu、Junbiao Chang、Yangjie WuDOI:10.1016/j.tet.2014.09.087日期:2014.11The first example of palladium-catalyzed protocol for the denitrated coupling of nitroarenes with sulfinates was developed, achieving aryl and heterocyclic sulfones in moderate to excellent yields. The cyclopalladated ferrocenylimine (I) exhibited highly catalytic activity for this transformation with low catalyst loading (0.75 mol %). The efficiency of this reaction was demonstrated by compatibility
-
Highly selective hydrogenation of halogenated nitroarenes over Ru/CN nanocomposites by <i>in situ</i> pyrolysis作者:Shengnan Yue、Xueguang Wang、Shaoting Li、Yao Sheng、Xiujing Zou、Xionggang Lu、Chunlei ZhangDOI:10.1039/d0nj02165b日期:——A highly chemoselective and recyclable ruthenium catalyst for the hydrogenation of halogenated nitroarenes has been prepared via the simple in situ calcination of a mixture of melamine, glucose and ruthenium trichloride. Superfine Ru particles (2.3 ± 0.3 nm) were obtained and highly dispersed in the nitrogen-doped carbon matrix. The Ru/CN catalyst smoothly transforms a variety of halogenated nitroarenes
表征谱图
-
氢谱1HNMR
-
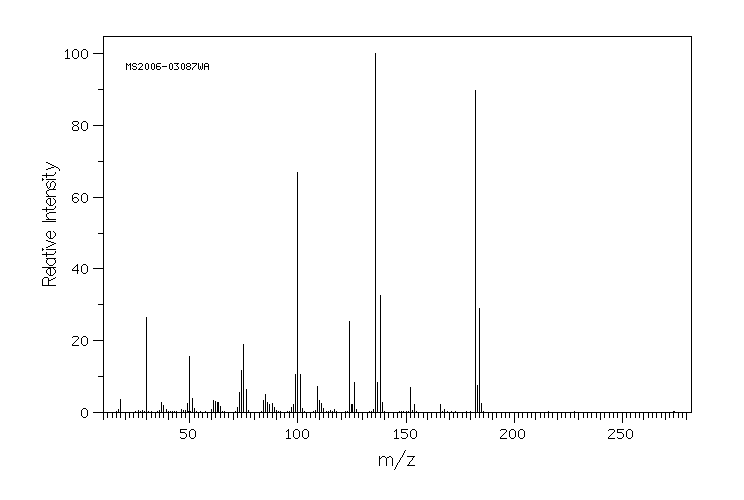
质谱MS
-
碳谱13CNMR
-
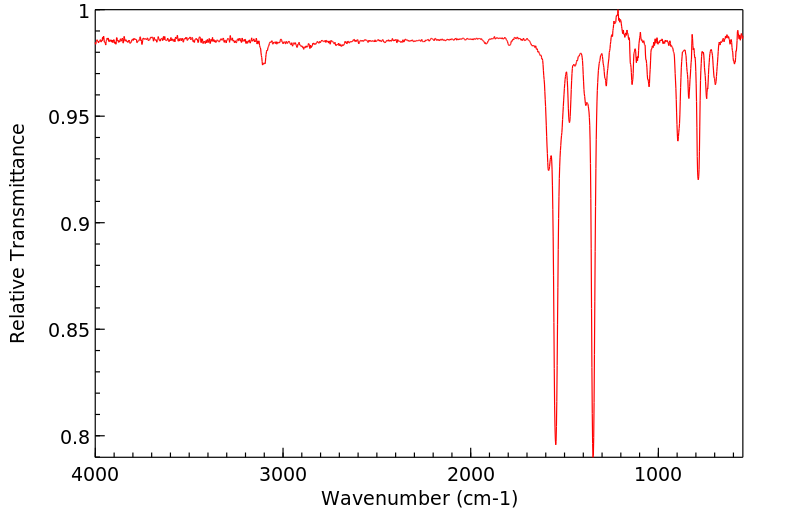
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫