4,5-二苯基咪唑 | 668-94-0
中文名称
4,5-二苯基咪唑
中文别名
4,5-二苯基-1,3-二氮唑;4,5-二甲苯-1H-咪唑;4,5-二苯基甘啉;4,5-二苯并咪唑;4,5-二苯基甘噁啉
英文名称
4,5-diphenyl-1H-imidazole
英文别名
4,5-Diphenylimidazole;4,5-bisphenyl-1H-imidazole;4,5-bis(phenyl)imidazole
CAS
668-94-0
化学式
C15H12N2
mdl
MFCD00005198
分子量
220.274
InChiKey
CPHGOBGXZQKCKI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:228-230 °C(lit.)
-
沸点:351.21°C (rough estimate)
-
密度:1.149
-
溶解度:溶于甲醇。
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:17
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:28.7
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933290090
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:存放在密封容器内,并置于阴凉、干燥处。存储地点须远离氧化剂。
SDS
模块 1. 化学品
1.1 产品标识符
: 4,5-二苯基咪唑
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C15H12N2
分子式
: 220.27 g/mol
分子量
组分 浓度或浓度范围
4,5-Diphenyl-1H-imidazole
-
化学文摘登记号(CAS 668-94-0
No.) 211-573-3
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 228 - 230 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强酸
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-溴-4,5-二苯基-1H-咪唑 2-bromo-4,5-diphenylimidazole 69045-24-5 C15H11BrN2 299.17 4,5-二苯咪唑啉酮 4,5-diphenyl-1,3-dihydro-imidazol-2-one 642-36-4 C15H12N2O 236.273 —— 2-Chlor-4,5-diphenylimidazol 49855-38-1 C15H11ClN2 254.719 4,5-二苯基-2-咪唑硫醇 2-mercapto-4,5-diphenylimidazole 2349-58-8 C15H12N2S 252.34 —— 2-vinylthio-4,5-diphenylimidazole 59282-91-6 C17H14N2S 278.378 5-氯-2,3-二苯基吡嗪 2-chloro-5,6-diphenylpyrazine 41270-66-0 C16H11ClN2 266.73 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4,4'-(1H-imidazole-4,5-diyl)-bis-aniline 46966-53-4 C15H14N4 250.303 —— 4,5-diphenyl-1H-imidazol-2-amine 37980-29-3 C15H13N3 235.288 2-溴-4,5-二苯基-1H-咪唑 2-bromo-4,5-diphenylimidazole 69045-24-5 C15H11BrN2 299.17 —— 4,5-bis(4-nitrophenyl)-1H-imidazole 92438-74-9 C15H10N4O4 310.269 苦杏碱 2,3,5,6-tetraphenylpyrazine 642-04-6 C28H20N2 384.48 —— 4,5-diphenyl-1-methyl-1H-imidazole 50609-88-6 C16H14N2 234.301 —— 5-<(4,5-diphenyl-1H-imidazol-2-yl)amino>-1-aminopentane 139772-91-1 C20H24N4 320.437 —— 4,5-Diphenylimidazol-2-carbonsaeure 101291-01-4 C16H12N2O2 264.283 —— N-<5-<(4,5-diphenyl-1H-imidazol-2-yl)amino>pentyl>-N-heptylamine 139772-92-2 C27H38N4 418.626 —— N-<5-<(4,5-diphenyl-1H-imidazol-2-yl)amino>pentyl>heptanamide 163318-67-0 C27H36N4O 432.609 1-乙基-4,5-二苯基咪唑 1-Ethyl-4,5-diphenyl-imidazol 1632-82-2 C17H16N2 248.327 1-乙烯基-4,5-二苯基咪唑 1-vinyl-4,5-diphenylimidazole 29878-11-3 C17H14N2 246.312 —— 2-((4-methoxyphenyl)-azo)-4,5-diphenylimidazole 51124-76-6 C22H18N4O 354.411 —— 2,4-Diphenyl-1-propylimidazole 1837-56-5 C18H18N2 262.354 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Purushothaman, E.; Pillai, V. N. Rajasekharan, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1989, vol. 28, # 1-11, p. 290 - 293摘要:DOI:
-
作为产物:描述:参考文献:名称:Watanabe, T.; Nishiyama, J.; Hirate, R., Journal of Heterocyclic Chemistry, 1983, vol. 20, p. 1277 - 1281摘要:DOI:
-
作为试剂:描述:参考文献:名称:一种4,5-二苯基咪唑啉的合成方法及用途摘要:一种有如下结构式的手性化合物合成方法:包括合成、分离和纯化,所述的合成是无水无氧条件下,用催化剂钯配合物1mol%做催化剂,称取偶苯酰0.5mmol及5.0g甲酸铵,放入250mL两口烧瓶中,加入100mL氯苯做溶剂,回流反应30小时后,柱层析分离,用石油醚/二氯甲烷(1/100)洗脱,将收集的最后组分点自然挥发,得单晶4,5‑二苯基咪唑啉:该手性化合物(Ⅰ)的用途,在二苯甲酮亚胺与三甲基硅腈反应中显示了一定的催化效果,其转化率达66%。公开号:CN111675629A
文献信息
-
Heme Oxygenase Inhibition by 2-Oxy-substituted 1-Azolyl-4-phenylbutanes: Effect of Variation of the Azole Moiety. X-Ray Crystal Structure of Human Heme Oxygenase-1 in Complex with 4-Phenyl-1-(1<i>H</i>-1,2,4-triazol-1-yl)-2-butanone作者:Gheorghe Roman、Mona N. Rahman、Dragic Vukomanovic、Zongchao Jia、Kanji Nakatsu、Walter A. SzarekDOI:10.1111/j.1747-0285.2009.00909.x日期:2010.15‐substituted triazoles, identically 3,5‐disubstituted triazoles, 5‐substituted‐1H‐ and 5‐substituted‐2H‐tetrazoles proved to be detrimental to the inhibition of HO, with a few exceptions. The azole‐dioxolanes and the azole‐alcohols derived from the active azole‐ketones were synthesized also, but these inhibitors were less active than the corresponding imidazole‐based analogs. The first reported X‐ray crystal为抑制血红素加氧酶(血红素加氧酶-1和血红素加氧酶-2),设计并合成了一系列的1-偶氮基-4-苯基-2-丁酮。用其他唑类取代咪唑导致发现了新型的1 H -1,2,4-三唑和1 H-四唑类抑制剂,与咪唑类铅抑制剂等效。具有2 H-四唑或1 H的抑制剂‐1,2,3-三唑作为药效基团的效力较低。通过各种吸电子或给电子的,小的或庞大的基团,在咪唑的2或4(5)位置被单取代,或在咪唑的4和5位置相同的解离,以及用阵列代替传统的咪唑药效基团3或5取代的三唑,3,5-二取代的三唑,5取代的1 H和5取代的2 H除少数例外,四唑类被证明对HO的抑制是有害的。还合成了由活性唑酮衍生的唑二氧杂戊环酮和唑醇,但这些抑制剂的活性低于相应的基于咪唑的类似物。首次报道了人类血红素加氧酶-1与1,2,4-三唑基抑制剂(即4-苯基-1-(1 H -1,2,4-三唑-1)的复合物的X射线晶体结构yl)-2-丁酮也已确定。该抑制剂通过三唑部分中的N
-
Non-prostanoid thromboxane A2 receptor antagonists with a dibenzoxepin ring system. 2作者:Etsuo Ohshima、Hitoshi Takami、Hideyuki Sato、Shinichiro Mohri、Hiroyuki Obase、Ichiro Miki、Akio Ishii、Shiro Shirakura、Akira Karasawa、Kazuhiro KuboDOI:10.1021/jm00096a017日期:1992.911-[2-(1-benzimidazolyl)ethylidene]-6,11-dihydrodibenz[b,e]oxep in-2- carboxylic acid derivatives and related compounds were synthesized and found to be potent TXA2/PGH2 receptor antagonists. Each compound synthesized was tested for its ability to displace [3H]U-46619 binding from guinea pig platelet TXA2/PGH2 receptors. Structure-activity relationship studies revealed that the following key elements were合成了一系列11- [2-(1-苯并咪唑基)亚乙基] -6,11-二氢二苯并[b,e] oxep in-2-羧酸衍生物及相关化合物,发现它们是有效的TXA2 / PGH2受体拮抗剂。测试了每种合成的化合物从豚鼠血小板TXA2 / PGH2受体置换[3H] U-46619结合的能力。构效关系研究表明,增强活性需要以下关键元素:(1)在二苯并二甲苯环系统的11位上的(E)-2-(1-苯并咪唑基)亚乙基侧链和(2)a在二苯并二氧杂戊环体系的2-位上的羧基。研究还表明,该系列化合物在豚鼠血小板中与TXA2 / PGH2受体的结合亲和力与人血小板中的相关性较弱。将取代基引入苯并咪唑部分是有效的,并且(E)-11- [2-(5,6-二甲基-1-苯并咪唑基)亚乙基]钠-6,11-二氢二苯并[b,e] oxepin-2 -羧酸一水合物(57)对人血小板TXA2 / PGH2受体的亲和力最高,K(i)值为1
-
Palladium-mediated synthesis of poly-fused heterocycles from Baylis–Hillman adducts作者:Hyun Seung Lee、Sung Hwan Kim、Saravanan Gowrisankar、Jae Nyoung KimDOI:10.1016/j.tet.2008.05.087日期:2008.7We synthesized various poly-fused heterocyclic compounds in good to moderate yields via the intramolecular Heck type reaction of Baylis–Hillman adducts modified with indole, imidazole, benzimidazole, and isatin.
-
[4 + 2]-Annulation of MBH-Acetates of Acetylenic Aldehydes with Imidazoles/Benzimidazoles To Access Imidazo[1,2-<i>a</i>]pyridines/Benzimidazo[1,2-<i>a</i>]pyridines作者:Chada Raji Reddy、Amarender Goud BurraDOI:10.1021/acs.joc.9b01118日期:2019.7.19developed to construct diversely substituted imidazo[1,2-a]pyridines and benzimidazo[1,2-a]pyridines in good to excellent yield. The advantage of this unique [4 + 2]-annulation lies in the employment of readily accessible starting materials, Morita–Baylis–Hillman acetates of acetylenic aldehydes as C4 synthons, and simple imidazoles or benzimidazoles as C2 synthons.
-
Sequential decarboxylative azide–alkyne cycloaddition and dehydrogenative coupling reactions: one-pot synthesis of polycyclic fused triazoles作者:Kuppusamy Bharathimohan、Thanasekaran Ponpandian、A Jafar Ahamed、Nattamai BhuvaneshDOI:10.3762/bjoc.10.321日期:——
Herein, we describe a one-pot protocol for the synthesis of a novel series of polycyclic triazole derivatives. Transition metal-catalyzed decarboxylative CuAAC and dehydrogenative cross coupling reactions are combined in a single flask and achieved good yields of the respective triazoles (up to 97% yield). This methodology is more convenient to produce the complex polycyclic molecules in a simple way.
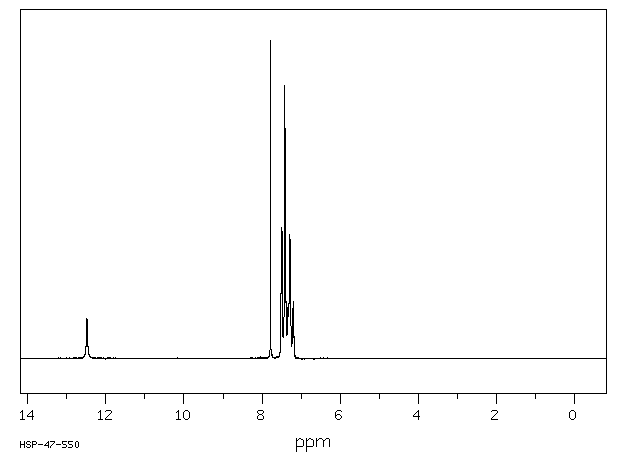
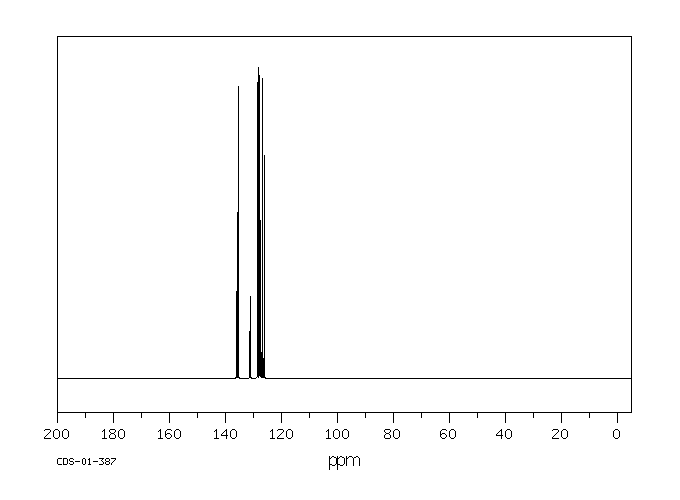
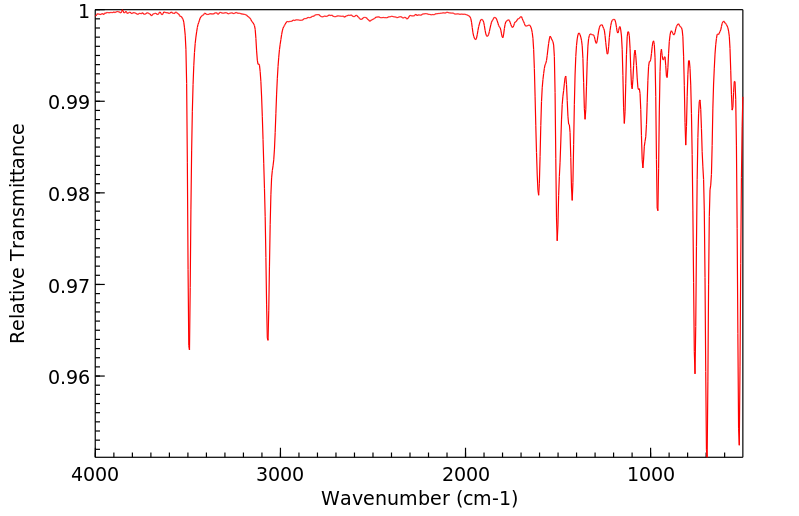
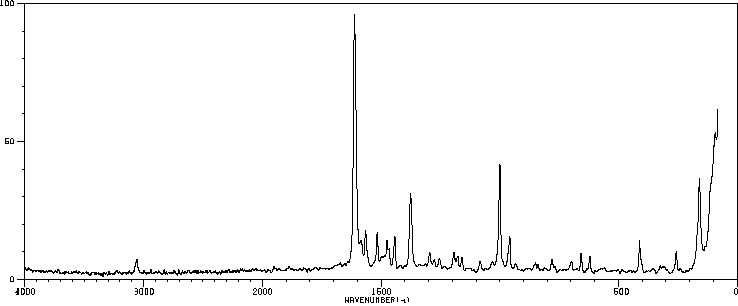
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)