乙烯基乙炔 | 689-97-4
中文名称
乙烯基乙炔
中文别名
——
英文名称
3-buten-1-yne
英文别名
vinylacetylene;1-Buten-3-yne;but-1-en-3-yne
CAS
689-97-4
化学式
C4H4
mdl
MFCD00053683
分子量
52.0758
InChiKey
WFYPICNXBKQZGB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:203 °C(Solv: water (7732-18-5))
-
沸点:3°C
-
密度:0.6800
-
物理描述:1-buten-3-yne appears as a colorless gas or liquid. Derived by the dimerization of acetylene. Used in the synthesis of neoprene and for other organic syntheses.
-
颜色/状态:Colorless gas or liquid
-
气味:acetylene-like odor
-
溶解度:In water, 1.79X10+3 mg/l @ 30 °C
-
蒸汽密度:1.80 (AIR= 1)
-
蒸汽压力:1,350 mm Hg @ 25 °C
-
分解:Very exothermic decomposition when heated. ... When heated to decomposition it emits acrid smoke and irritating fumes.
-
折光率:Index of refraction: 1.4161 @ 1 °C/D
-
保留指数:407.15;404;410
-
稳定性/保质期:
-
在空气中非常容易氧化成爆炸性的过氧化物,易发生加成反应和聚合反应。
-
本品有毒。对人体有刺激和麻醉作用。中毒后会引起头痛、眩晕、咽喉干燥、腿部无力,有时还会呕吐,并能引起神经、肝等疾病。空气中的最高容许浓度为0.01mg/L。
-
存在于烟气中。
-
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
毒理性
神经毒素 - 急性溶剂综合征
Neurotoxin - Acute solvent syndrome
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
LC50(小鼠)= 97,200 毫克/立方米/2小时
LC50 (mice) = 97,200 mg/m3/2h
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
基本治疗:建立专利气道。如有必要,进行吸痰。观察呼吸不足的迹象,如有必要,协助通气。通过非循环呼吸面罩以10至15升/分钟的速度给予氧气。监测休克并视需要进行治疗……预见并治疗癫痫发作……对于眼睛污染,立即用水冲洗眼睛。在运输过程中,用生理盐水连续冲洗每只眼睛……不要使用催吐剂。对于误食,如果患者能吞咽,有强烈的干呕反射,且不流口水,则用水冲洗口腔,并给予5毫升/千克,最多200毫升的水进行稀释。给予活性炭……/脂肪烃及其相关化合物/
Basic treatment: Establish a patent airway. Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with normal saline during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool. Administer activated charcoal ... . /Aliphatic hydrocarbons and related compounds/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
高级治疗:对于失去意识或呼吸暂停的患者,考虑进行口咽或鼻咽气管插管以控制气道。使用气囊-阀-面罩装置的正压通气技术可能有益。监测心率和必要时治疗心律失常。开始静脉输液,使用D5W/SRP:“保持通路开放”,最低流量/。如果出现低血容量的迹象,使用乳酸钠林格液。注意液体过载的迹象。考虑使用药物治疗肺水肿。用安定(安定)治疗癫痫。使用丙美卡因氢氯化物协助眼部冲洗。/脂肪烃及其相关化合物/
Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious or in respiratory rest. Positive pressure ventilation techniques with a bag-valve-mask device may be beneficial. Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start an IV with D5W /SRP: "To keep open", minimal flow rate/. Use lactated Ringer's if signs of hypovolemia are present. Watch for signs of fluid overload. Consider drug therapy for pulmonary edema ... . Treat seizures with diazepam (Valium) ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Aliphatic hydrocarbons and related compounds/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
LC50小鼠吸入97,200毫克/立方米/2小时
LC50 Mouse inhalation 97,200 mg/cu m/2 hr
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:2.1
-
危险品标志:Xn
-
危险品运输编号:1954
-
包装等级:Z01
-
危险类别:2.1
-
安全说明:S24/25,S36
-
危险类别码:R20/21/22
-
储存条件:库房应保持通风、低温和干燥的环境。需采用密闭方式存储,并将之与其他氧化剂和酸类分开存放。由于容易生成过氧化物,不宜长期储存。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 乙烯基乙炔 |
| 化学品英文名称: | Vinyl acetylene;Buten-3-yne |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 689-97-4 |
| 分子式: | C 4 H 4 |
| 分子量: | 52.04 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:乙烯基乙炔 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第2.1类 易燃气体 |
| 侵入途径: | 吸入 |
| 健康危害: | 急性中毒的表现:头痛、眩晕、腿无力、颌关节痛、出汗、咽喉干燥,有时有恶心、呕吐及腹泻。长时间接触后,可发生神经衰弱综合征,低血压等。 |
| 环境危害: | 对环境有危害,对大气可造成污染 |
| 燃爆危险: | 本品易燃。 |
| 第四部分:急救措施 |
| 皮肤接触: | |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水冲洗,至少15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。对症治疗。 |
| 食入: |
| 第五部分:消防措施 |
| 危险特性: | 与空气混合能形成爆炸性混合物。遇明火、高热极易燃烧爆炸。在空气中非常容易氧化生成过氧化物, 受热或撞击、甚至轻微摩擦即发生爆炸。能与浓硫酸、发烟硝酸猛烈反应, 甚至发生爆炸。在精馏操作过程中, 易发生自聚, 引起事故, 应加阻聚剂。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 切断气源。若不能立即切断气源,则不允许熄灭正在燃烧的气体,喷水冷却容器,可能的话将容器从火场移至空旷处。雾状水、泡沫、二氧化碳。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | <-5 |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | 2 |
| 爆炸上限[%(V/V)]: | 100 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 迅速撤离泄漏污染区人员至上风处,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防静电工作服。从上风处进入现场。尽可能切断泄漏源。用工业覆盖层或吸附/ 吸收剂盖住泄漏点附近的下水道等地方,防止气体进入。如无危险,就地燃烧,同时喷雾状水使周围冷却,以防其它可燃物着火。或用管路导至炉中、凹地焚之。漏气容器要妥善处理,修复、检验后再用。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作。密闭操作,提供良好的自然通风条件。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防静电工作服,戴防化学品手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止气体泄漏到工作场所空气中。避免与氧化剂、酸类、氧接触。在传送过程中,钢瓶和容器必须接地和跨接,防止产生静电。搬运时轻装轻卸,防止钢瓶及附件破损。配备相应品种和数量的消防器材及泄漏应急处理设备。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。库温不宜超过30℃。保持容器密封。应与氧化剂、酸类、氧等分开存放,切忌混储。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中国MAC:未制订标准前苏联MAC:20mg/m3 美国TLV-TWA:未制订标准美国TL |
| 监测方法: | |
| 工程控制: | 密闭操作。提供良好的自然通风条件。 |
| 呼吸系统防护: | 高浓度环境中,佩带供气式呼吸器。 |
| 眼睛防护: | 一般不需要特殊防护,高浓度接触时可戴化学安全防护眼镜。 |
| 身体防护: | 穿防静电工作服。 |
| 手防护: | 必要时戴防护手套。 |
| 其他防护: | 工作现场严禁吸烟。避免高浓度吸入。进入罐或其它高浓度区作业,须有人监护。 |
| 第九部分:理化特性 |
| 外观与性状: | 气态(常温) |
| pH: | |
| 熔点(℃): | -118 |
| 沸点(℃): | 5 |
| 相对密度(水=1): | 0.71 |
| 相对蒸气密度(空气=1): | 1.8 |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | 16.8 |
| 临界压力(MPa): | 5.035 |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | <-5 |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | 100 |
| 爆炸下限%(V/V): | 2 |
| 分子式: | C 4 H 4 |
| 分子量: | 52.04 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | |
| 主要用途: | 在工业上是重要的烯炔烃化合物,用于制备合成橡胶的单体2-氯丁二烯-[1,3]等。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、酸类、氧、卤素。 |
| 避免接触的条件: | 受热、光照。 |
| 聚合危害: | 能发生 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50: LC50:小鼠:97.2mg/L(2小时) |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 21060 |
| UN编号: | 1954 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 采用刚瓶运输时必须戴好钢瓶上的安全帽。钢瓶一般平放,并应将瓶口朝同一方向,不可交叉;高度不得超过车辆的防护栏板,并用三角木垫卡牢,防止滚动。运输时运输车辆应配备相应品种和数量的消防器材。装运该物品的车辆排气管必须配备阻火装置,禁止使用易产生火花的机械设备和工具装卸。严禁与氧化剂、酸类、氧等混装混运。夏季应早晚运输,防止日光曝晒。中途停留时应远离火种、热源。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。铁路运输时要禁止溜放。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 3 |
| MSDS修改日期: | 年月日 |
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-戊烯-3-炔 pent-1-en-3-yne 646-05-9 C5H6 66.1026 4-氯-丁-1-烯-3-炔 4-Chlorbutenin 40589-38-6 C4H3Cl 86.5208 —— 1-Iod-3-buten-1-in 40589-39-7 C4H3I 177.972 4-溴丁-1-烯-3-炔 4-bromo-but-1-en-3-yne 40083-64-5 C4H3Br 130.972
反应信息
-
作为反应物:参考文献:名称:Chemical process and product摘要:公开号:US02366311A1
-
作为产物:描述:1,3-二氯-2-丁烯 在 potassium hydroxide 作用下, 以 ethylene glycol tert-buty ether 、 乙二醇 为溶剂, 反应 2.0h, 生成 乙烯基乙炔参考文献:名称:(±)-SDEF 678 代谢物和 (±)-Speciosins AC 的首次合成摘要:环氧醌天然产物 SDEF 678 代谢物、speciosin A、speciosin B 和 speciosin C 首次通过基于钯催化偶联的卤素取代的 1,4-苯醌单缩酮(Diels– Alder/retro-Diels-Alder 序列和非对映选择性环氧化过程。DOI:10.1002/ejoc.201001402
-
作为试剂:参考文献:名称:丁基锂诱导的pent-3-en-1-yne二聚体及相关添加摘要:的烯炔HCCCHCHCH 2 - [R(RH,Me中的OME,NME 2 SME),被转换成二聚物通过用稍微超过两个当量的丁基锂。所述ž configuration占优势在水性workup后得到的二聚体。锂化烯炔(RH,Me中,SME)和乙炔基化合物LiCCCH 2 - [R(RSMe的,C 6 H ^ 5)以类似的方式来LiCCCHCH的双键加2。DOI:10.1016/0022-328x(91)86132-a
文献信息
-
Regioselective Catalytic and Stepwise Routes to Bulky, Functional-Group-Appended, and Luminescent 1,2-Azaborinines作者:Marius Schäfer、Julian Schäfer、Rian D. Dewhurst、William C. Ewing、Mirjam Krahfuß、Maximilian W. Kuntze-Fechner、Marius Wehner、Christoph Lambert、Holger BraunschweigDOI:10.1002/chem.201600653日期:2016.6.13The regioselective syntheses of 1,2‐azaborinines is achieved using an unsymmetrical iminoborane through both catalytic and stepwise modular routes. The 1,2‐azaborinine ring can be selectively functionalized in the 4‐ and/or 6‐position through control of the stepwise reaction sequence, allowing access to vinyl‐functionalized and redox‐active, luminescent, donor‐functionalized 1,2‐azaborinines. The electrochemistry
-
Asymmetric Synthesis of 3-Methyleneindolines via Rhodium(I)-Catalyzed Alkynylative Cyclization of <i>N</i>-(<i>o</i>-Alkynylaryl)imines作者:Shi-Yi Yuan、Qi-Qi Yan、Dan Wang、Ting-Ting Dan、Long He、Cheng-Yu He、Wen-Dao Chu、Quan-Zhong LiuDOI:10.1021/acs.orglett.1c01518日期:2021.6.18The first asymmetric synthesis of 3-methyleneindolines from alkynyl imines has been developed via a rhodium-catalyzed tandem process: regioselective alkynylation of the internal alkynes and subsequent intramolecular addition to the imines. The reaction proceeded with unconventional chemoselectivity and provided 3-methyleneindolines with good yields (up to 82% yield) and high enantioselectivities (up
-
Reaction Rate and Isomer-Specific Product Branching Ratios of C<sub>2</sub>H + C<sub>4</sub>H<sub>8</sub>: 1-Butene, <i>cis</i>-2-Butene, <i>trans</i>-2-Butene, and Isobutene at 79 K作者:Jordy Bouwman、Martin Fournier、Ian R. Sims、Stephen R. Leone、Kevin R. WilsonDOI:10.1021/jp403637t日期:2013.6.20rate constants are (1.9 ± 0.5) × 10–10, (1.7 ± 0.5) × 10–10, (2.1 ± 0.7) × 10–10, and (1.8 ± 0.9) × 10–10 cm3 s–1 for the reaction of C2H with 1-butene, cis-2-butene, trans-2-butene, and isobutene, respectively. Bimolecular rate constants for 1-butene and isobutene compare well to values measured previously at 103 K using C2H chemiluminescence. Photoionization spectra of the reaction products are measured在79 K的Laval喷嘴膨胀条件下,通过激光光解-真空紫外质谱法研究了C 2 H自由基与C 4 H 8异构体1-丁烯,顺式-2-丁烯,反式-2-丁烯和异丁烯的反应。通过在伪一级条件下测量反应产物种类的形成速率与反应物密度的函数,可以得到双分子反应速率常数。所述速率常数(1.9±0.5)×10 -10,(1.7±0.5)×10 -10,(2.1±0.7)×10 -10,和(1.8±0.9)×10 -10厘米3个小号-1为C 2的反应H分别与1-丁烯,顺式-2-丁烯,反式-2-丁烯和异丁烯一起。1-丁烯和异丁烯的双分子速率常数与先前使用C 2 H化学发光法在103 K下测得的值相比较。测量反应产物的光电离光谱,并将其拟合为贡献异构体的电离光谱。结合绝对电离截面,这些拟合可提供异构体拆分的产物分支馏分。C 2 H和1-丁烯之间的反应生成乙烯基乙炔形式的(65±10)%C 4 H 4和4-戊烯-1-炔形式的(35±10)%C
-
Syntheses with Organoboranes. X. Monohydroboration of Conjugated Enynes with Catecholborane Catalyzed by Nickel(II) Chloride Complexes with Diphosphines作者:Marek Zaidlewicz、Jerzy MellerDOI:10.1135/cccc19991049日期:——
The monohydroboration of representative conjugated enynes - but-1-en-3-yne (
1 ), 2-methylbut-1-en-3-yne (2 ), pent-3-en-1-yne (3 ), hex-1-en-3-yne (4 ), 2-methyl-4-phenylbut-1-en-3-yne (5 ), 4-methyl-1-phenylpent-3-en-1-yne (6 ) and 1-ethynylcyclohex-1-ene (7 ), with catecholborane in the presence of the nickel(II) chloride complex with 1,2-bis(diphenylphosphino)ethane gave the corresponding 1,3-dien-1-yl organoboranes in 54-87% yield. High regioselectivity of the addition leading to the boron atom at the 4-position of the 1-en-3-yne system was observed for1 ,2 ,3 ,6 and7 . -
Cascade Reactions of β-Amino-Substituted α,β-Unsaturated Fischer Carbene Complexes with 1,5-Dien-3-ynes as a Convenient Access to Ring-Annelated Benzene Derivatives作者:Yao-Ting Wu、Mathias Noltemeyer、Armin de MeijereDOI:10.1002/ejoc.200500130日期:2005.7achieve good chemical yields. This new cascade reaction of Fischer carbene complexes provides a direct route to trindanone analogues under milder conditions than traditional methods, and is compatible with more functionalities. Compounds 14 and 15 with steroid-like skeletons were thus prepared in 54–77 % yields (4 examples) from complex 1-iPr and the bicyclic alkyne 2. Hexacycles 17 and 18 were accessible
表征谱图
-
氢谱1HNMR
-
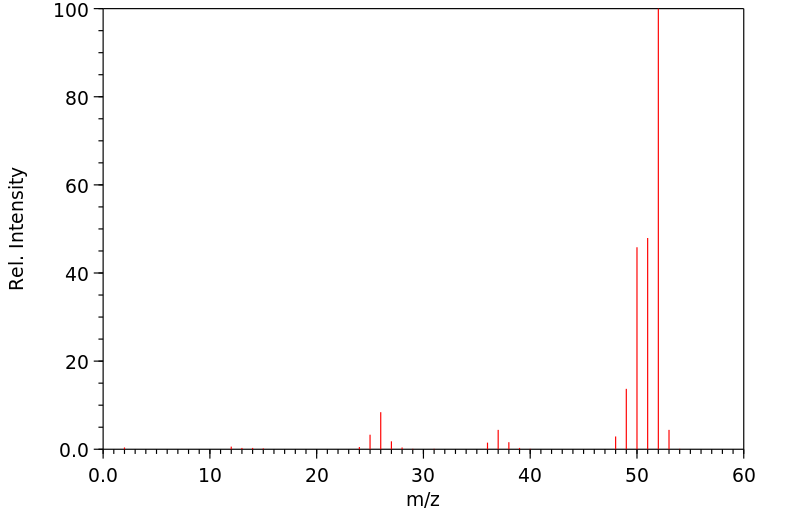
质谱MS
-
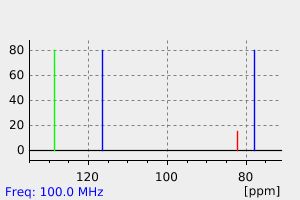
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-