N,N-二甲基三氟乙酰胺 | 1547-87-1
中文名称
N,N-二甲基三氟乙酰胺
中文别名
2,2,2-三氟-N,N-二甲基乙酰胺;N,N'-二甲基三氟乙酰胺
英文名称
N,N-dimethyl-2,2,2-trifluoroacetamide
英文别名
2,2,2-trifluoro-N,N-dimethylacetamide;N,N-dimethyltrifluoroacetamide;trifluoroacetyl dimethylamine
CAS
1547-87-1
化学式
C4H6F3NO
mdl
——
分子量
141.093
InChiKey
WXBWKMLIVXELSF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:132 °C
-
密度:1.23
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:9
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2924199090
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P210,P233,P240,P241+P242+P243,P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313,P403+P235,P501
-
危险品运输编号:1993
-
危险性描述:H225,H315,H319
-
储存条件:请将贮藏器保持密封,并存放在阴凉干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2,2,2-Trifluoro-N,N-dimethylacetamide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,2,2-Trifluoro-N,N-dimethylacetamide
CAS number: 1547-87-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C4H6F3NO
Molecular weight: 141.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2,2,2-Trifluoro-N,N-dimethylacetamide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,2,2-Trifluoro-N,N-dimethylacetamide
CAS number: 1547-87-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C4H6F3NO
Molecular weight: 141.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N,N-Dimethyl-2-trifluor-ethylamin 819-06-7 C4H8F3N 127.109
反应信息
-
作为反应物:参考文献:名称:Fluorocarbon Nitrogen Compounds. I. Perfluorocarbamic Acid Derivatives, Amides and Oxazolidines1摘要:DOI:10.1021/ja01602a046
-
作为产物:描述:参考文献:名称:Cheburkov,Yu.A. et al., Doklady Chemistry, 1966, vol. 169, # 1, p. 677 - 680摘要:DOI:
-
作为试剂:描述:4'-甲基乙酰乙酰苯胺 、 苯甲醛 在 哌啶 、 N,N-二甲基三氟乙酰胺 、 三氟甲磺酸酐 、 溶剂黄146 、 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 反应 4.0h, 生成 3-acetyl-6-methyl-4-phenylquinolin-2(1H)-one参考文献:名称:Triflic酸酐促进N-芳基肉桂酸酯的分子内环化:访问多取代的喹啉2(1H)-一。摘要:摘要 通过在温和的条件下在N,N-二甲基三氟乙酰胺(DTA)中由三氟甲磺酸酐促进的易于获得的N-芳基肉桂酸酯的分子内环化作用,开发了一种简便,有效的多取代喹啉2(1 H)-酮合成方法。 通过在温和的条件下在N,N-二甲基三氟乙酰胺(DTA)中由三氟甲磺酸酐促进的易于获得的N-芳基肉桂酸酯的分子内环化作用,开发了一种简便,有效的多取代喹啉2(1 H)-酮合成方法。DOI:10.1055/s-0036-1590821
文献信息
-
Zirconium-hydride-catalyzed site-selective hydroboration of amides for the synthesis of amines: Mechanism, scope, and application作者:Bo Han、Jiong Zhang、Haijun Jiao、Lipeng WuDOI:10.1016/s1872-2067(21)63853-6日期:2021.11diverse amines. Various readily reducible functional groups, such as esters, alkynes, and alkenes, were well tolerated. Furthermore, the methodology was extended to the synthesis of bio- and drug-derived amines. Detailed mechanistic studies revealed a reaction pathway entailing aldehyde and amido complex formation via an unusual C–N bond cleavage-reformation process, followed by C–O bond cleavage.
-
一种3`,5`-二氯-2,2,2-三氟苯乙酮的合成方法
-
一种酰胺化合物还原制备胺类化合物的方法
-
Fluorescent Dyes with Large Stokes Shifts Based on Benzo[1,2‐d:4,5‐d']bis([1,3]dithiole) (“S <sup>4</sup> ‐DBD Dyes”)作者:Pablo Wessig、Daniel Freyse、David Schuster、Alexandra KellingDOI:10.1002/ejoc.202000093日期:2020.3.22Replacing oxygen by sulfur atoms substantially changes the photophysical behavior of DBD dyes. While fluorescence quantum yields and lifetimes are decreased, absorption and emission wavelength markedly red‐shifted. The very large Stokes shifts should be especially emphasized. A series of S4‐DBD dyes with different electron withdrawing groups were prepared enabling a broad range of emission wavelength
-
Mild catalytic deoxygenation of amides promoted by thorium metallocene作者:Sayantani Saha、Moris S. EisenDOI:10.1039/d0dt02770g日期:——organoactinide-catalyzed (Cp*2ThMe2) hydroborated reduction of a wide range of tertiary, secondary, and primary amides to the corresponding amines/amine–borane adducts via deoxygenation of the amides is reported herein. The catalytic reactions proceed under mild conditions with low catalyst loading and pinacolborane (HBpin) concentration in a selective fashion. Cp*2ThMe2 is capable of efficiently catalysing
表征谱图
-
氢谱1HNMR
-
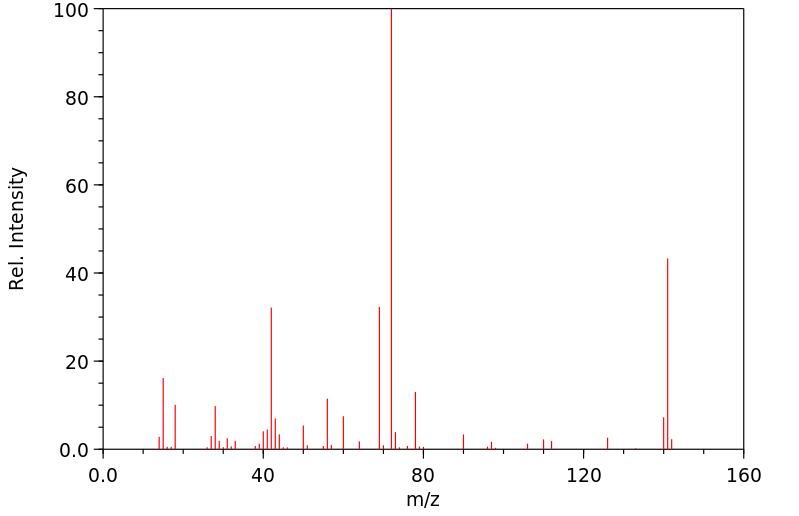
质谱MS
-
碳谱13CNMR
-
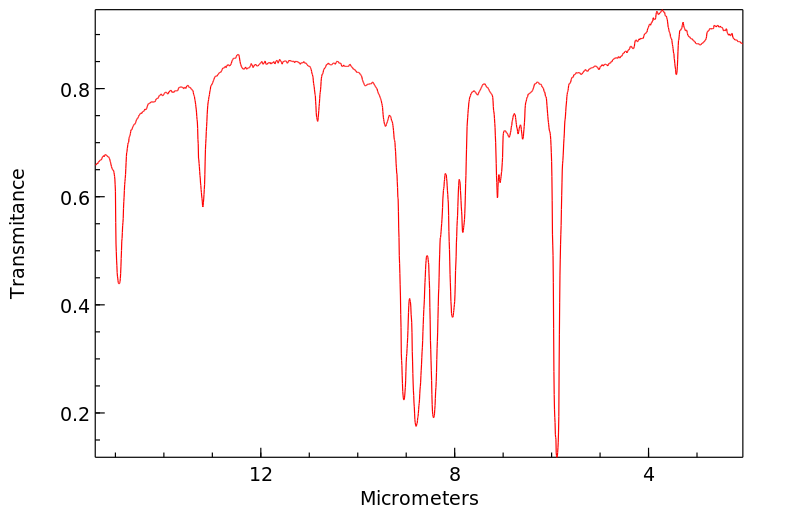
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸