2-(苯基磺酰)苯乙酮 | 3406-03-9
中文名称
2-(苯基磺酰)苯乙酮
中文别名
2-苯磺酰基苯乙酮;2-苯磺酰乙酮
英文名称
2-(phenylsulfonyl)acetophenone
英文别名
phenacylphenyl sulfone;1-phenyl-2-(phenylsulfonyl)ethan-1-one;2-benzenesulfonylacetophenone;1-phenyl-2-(phenylsulfonyl)ethanone;2-(benzenesulfonyl)-1-phenylethanone
CAS
3406-03-9
化学式
C14H12O3S
mdl
MFCD00025043
分子量
260.313
InChiKey
DREVPGKOIZVPQV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93-95 °C(lit.)
-
沸点:373.57°C (rough estimate)
-
密度:1.2959 (rough estimate)
-
溶解度:溶于苯
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:18
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:59.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914700090
-
储存条件:常温下应避光存放在通风干燥处,并密封保存。
SDS
| Name: | 2-(Phenylsulfonyl)Acetophenone 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 3406-03-9 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3406-03-9 | 2-(Phenylsulfonyl)Acetophenone | 99% | 222-292-0 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3406-03-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 93-95 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H12O3S
Molecular Weight: 260.1422
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3406-03-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-(Phenylsulfonyl)Acetophenone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 3406-03-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3406-03-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3406-03-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ω-bromo-ω-phenylsulfonylacetophenone 23128-59-8 C14H11BrO3S 339.21 苯甲酰甲基苯基硫醚 1-phenyl-2-(phenylthio)ethanone 16222-10-9 C14H12OS 228.315 —— β-phenyl-β-hydroxyethyl phenyl sulfone 51755-92-1 C14H14O3S 262.329 [2-(苯磺酰基)-1-碘乙基]苯 1-Benzenesulphonyl-2-iodo-2-phenylethane 77144-78-6 C14H13IO2S 372.227 —— ((2-phenylallyl)sulfonyl)benzene 74866-37-8 C15H14O2S 258.341 —— (Z)-2-(benzenesulfonyl)-3-ethoxy-1-phenylprop-2-en-1-one —— C17H16O4S 316.4 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-phenyl-2-(phenylsulfonyl)propan-1-one 27839-91-4 C15H14O3S 274.34 1-苯基-2-(苯基磺酰基)乙烷 1-phenyl-2-(phenylsulfonyl)ethane 27846-25-9 C14H14O2S 246.33 —— 2-(Benzenesulfonyl)-1-phenyl-2-propen-1-one 87537-08-4 C15H12O3S 272.324 —— ω-bromo-ω-phenylsulfonylacetophenone 23128-59-8 C14H11BrO3S 339.21 —— 2-fluoro-1-phenyl-2-(phenylsulfonyl)ethanone 177174-40-2 C14H11FO3S 278.304 —— 2-methyl-1-phenyl-2-phenylsulfonyl-1-propanone 126078-18-0 C16H16O3S 288.367 —— 1,3-diphenyl-2-phenylsulfonyl-1-propanone 34544-98-4 C21H18O3S 350.438 —— 1-phenyl-2-(phenylsulfonyl)butan-1-one 131454-22-3 C16H16O3S 288.367 —— 2-benzenesulfonyl-2,2-dibromo-1-phenyl-ethanone 23128-58-7 C14H10Br2O3S 418.106 —— β-phenyl-β-hydroxyethyl phenyl sulfone 51755-92-1 C14H14O3S 262.329 —— 1-phenyl-2-(phenylsulfonyl)ethanol —— C14H14O3S 262.329 —— (S)-1-phenyl-2-(phenylsulfonyl)ethanol 224634-51-9 C14H14O3S 262.329 2-(苯基磺酰基)重氮苯乙酮 Benzoyl(phenylsulfonyl)diazomethan 28322-50-1 C14H10N2O3S 286.311 —— 2,2-difluoro-1-phenyl-2-(phenylsulfonyl)ethan-1-one 734-18-9 C14H10F2O3S 296.294 —— 2-benzenesulfonyl-1-phenyl-pent-4-en-1-one 104869-91-2 C17H16O3S 300.378 —— (1-Ethoxy-1-phenylsulfonylmethyl)(phenyl)keton 57928-22-0 C16H16O4S 304.367 —— 1-phenyl-2-(phenylsulfonyl)hexane-1,5-dione 1191924-90-9 C18H18O4S 330.405 —— 1-phenyl-2-(phenylsulfonyl)ethan-1-one oxime 908814-02-8 C14H13NO3S 275.328 —— [2-(Benzenesulfonyl)-1-phenylethylidene]hydrazine —— C14H14N2O2S 274.343 —— 1-benzenesulfonyl-1-benzoyl-2-(N,N-dimethylamino)ethene 108289-88-9 C17H17NO3S 315.393 —— 1-(phenylsulfonyl)cyclopropyl phenyl ketone 119090-44-7 C16H14O3S 286.351 —— 1-phenyl-2-(phenylsulfonyl)-heptane-1,5-dione 1191925-04-8 C19H20O4S 344.431 —— 2-Benzoyl-2-phenylsulfonylvinyl-ethyl-ether 32083-40-2 C17H16O4S 316.378 —— (Z)-2-(benzenesulfonyl)-3-ethoxy-1-phenylprop-2-en-1-one —— C17H16O4S 316.4 —— (E)-1,5-diphenyl-2-(phenylsulfonyl)pent-4-en-1-one —— C23H20O3S 376.476 —— 2-(Benzenesulfonyl)-1,5-diphenylpent-4-en-1-one 606138-60-7 C23H20O3S 376.476 —— N-(2-benzoyl-2-phenylsulfonylethyl)piperidine 97039-09-3 C20H23NO3S 357.474 —— 5-benzenesulfonyl-6-oxo-6-phenyl-hex-2-enoic acid ethyl ester —— C20H20O5S 372.442 —— (E)-1,3-diphenyl-2-(phenylsulfonyl)prop-2-en-1-one 19232-53-2 C21H16O3S 348.422 —— 1,3-diphenyl-2-(phenylsulfonyl)-2-propen-1-one 150007-79-7 C21H16O3S 348.422 —— α-phenylsulfonyl chalcone 19232-53-2 C21H16O3S 348.422 —— 4-methyl-1-phenyl-2-(phenylsulfonyl)hexane-1,5-dione 1191925-00-4 C19H20O4S 344.431 —— 1,5-diphenyl-2,4-bis(phenylsulfonyl)pentane-1,5-dione 3406-04-0 C29H24O6S2 532.638 —— 1-phenyl-2-benzenesulfonyl-3-(4-fluorophenyl)-prop-2-en-1-one 105966-78-7 C21H15FO3S 366.413 2-丙烯-1-酮,3-(4-氟苯基)-1-苯基-2-(苯磺酰)-,(E)- 2-(Benzenesulfonyl)-3-(4-fluorophenyl)-1-phenylprop-2-en-1-one 105966-78-7 C21H15FO3S 366.4 —— 2-Benzenesulfonyl-1-phenyl-2-(p-tolyl-hydrazono)-ethanone 35458-23-2 C21H18N2O3S 378.452 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:2-(苯基磺酰)苯乙酮 在 sodium hydroxide 、 双氧水 、 potassium carbonate 、 potassium bromide 作用下, 以 丙酮 、 乙腈 为溶剂, 反应 7.5h, 生成 ((1-bromoethyl)sulfonyl)benzene参考文献:名称:Chemoselective mono halogenation of β-keto-sulfones using potassium halide and hydrogen peroxide; synthesis of halomethyl sulfones and dihalomethyl sulfones摘要:The synthesis of alpha-halo beta-keto-sulfones using potassium halide and hydrogen peroxide as a chemoselective mono halogenation reagent and the synthesis of alpha, alpha-symmetrical and asymmetrical dihalo beta-keto-sulfones and alpha-halo, alpha-alkyl and beta-keto-sulfones is described. Base induced cleavage of alpha-halo beta-keto-sulfones, alpha,alpha-dihalo beta-keto-sulfones, and alpha-halo, alpha-alkyl beta-keto-sulfones afforded the corresponding halomethyl sulfones, dihalomethyl sulfones and haloalkyl sulfones. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2006.11.129
-
作为产物:描述:phosphoric acid diethyl ester-(1-phenyl-vinyl ester) 在 水 、 氧气 作用下, 以 二氯甲烷 为溶剂, 反应 3.5h, 生成 2-(苯基磺酰)苯乙酮参考文献:名称:水中活化烯烃的无催化剂双官能化:β-酮基硫化物和砜的高效合成摘要:直接合成一系列β-酮硫醚和β-酮砜的化学合成中,有效的策略是活化烯烃的双官能团化。由O 2介导的转化在水中顺利进行且没有任何催化剂。该方法的显着优势包括温和的反应条件,纯化简便和克级合成,这突显了该方法的实际实用性。DOI:10.1002/chem.201603041
文献信息
-
NAD(P)<sup>+</sup>–NAD(P)H Models. 65. Photochemical Reductive Desulfonylation of β-Keto Sulfones with Hantzsch Ester作者:Masayuki Fujii、Kaoru Nakamura、Hideyuki Mekata、Shinzaburo Oka、Atsuyoshi OhnoDOI:10.1246/bcsj.61.495日期:1988.2A new procedure for the reductive desulfonylation of β-keto sulfones is described. The reaction proceeds under a photochemical conditions with the Hantzsch ester in pyridine in the presence of ruthenium(II). Various functional groups are unaffected under the reaction conditions. Application of the procedure to the syntheses of certain natural products is also described.
-
Base-mediated tandem sulfonylation and oximation of alkenes in water作者:Bin Wang、Lin Tang、Liyan Liu、Yanan Li、Yu Yang、Zhiyong WangDOI:10.1039/c7gc03051g日期:——
A base-mediated bifunctionalization of alkenes for the synthesis of α-sulfonylethanone oximes was developed in water under metal-free conditions. This reaction features a wide substrate scope and facile starting materials to afford the desired products in high yields.
-
3,3-Sigmatropic Rearrangements Involving N−O Bond-Cleavage of Enehydroxylamine Derivatives作者:Lucinda V. Reis、Ana M. Lobo、Sundaresan Prabhakar、Mariana P. DuarteDOI:10.1002/1099-0690(200301)2003:1<190::aid-ejoc190>3.0.co;2-w日期:2003.1good to excellent yields, substances that in general undergo 3,3-sigmatropic rearrangements either spontaneously or upon heating. In those cases in which such reactions failed, addition of sodium hydride was found to induce the transformation. A study of the rearrangement by use of deuterium-labelled compounds showed that no crossover occurs, indicating the intramolecular nature of the process. The
-
Sulfated tungstate/dioxygen: a new catalytic system for oxysulfonylation of styrenes to form β-keto sulfones作者:Ganesh D. Wagh、Snehalata B. Autade、Raghavendra V. Kulkarni、Krishnacharya G. AkamanchiDOI:10.1039/d0nj01763a日期:——A new system for synthesis of a wide range of β-keto sulfones using sulfated tungstate as a heterogeneous catalyst and oxygen as an environmentally benign oxidant with aryl hydrazides and styrenes as reacting counterparts has been developed. The preliminary experimental results support the involvement of free radical species. Thus, aryl sulfonyl free radicals, generated by oxidation of aryl sulfonyl
-
Copper-Catalyzed Oxidative Trifunctionalization of Olefins: An Access to Functionalized β-Keto Thiosulfones作者:Shuai Huang、Nuligonda Thirupathi、Chen-Ho Tung、Zhenghu XuDOI:10.1021/acs.joc.8b01161日期:2018.8.17Aerobic oxidative trifunctionalization of olefins for the synthesis of functionalized β-keto thiosulfones has been described. The transformation proceeds through molecular oxygen activation under copper catalysis and forms the two new C–S bonds in a single operation using mild conditions. A novel Cu-catalyzed sulfonyl radical addition/oxidation/funtionalization relay mechanism was proposed for the
表征谱图
-
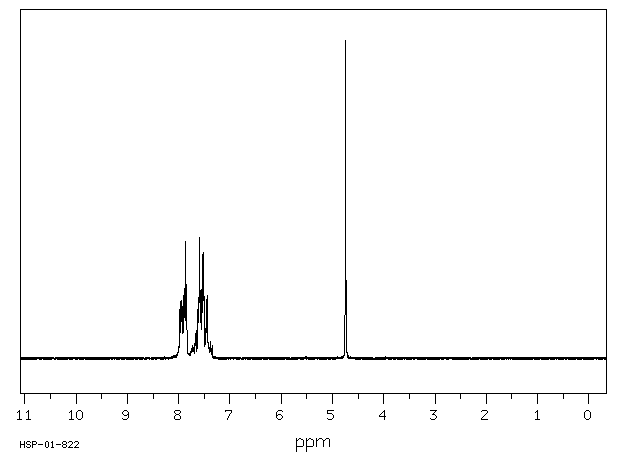
氢谱1HNMR
-
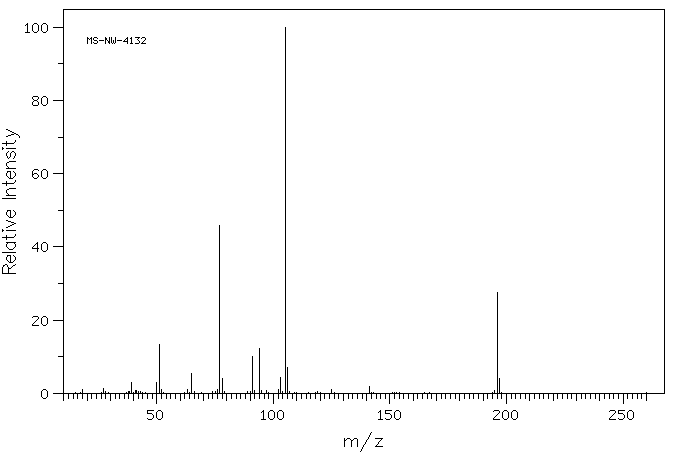
质谱MS
-
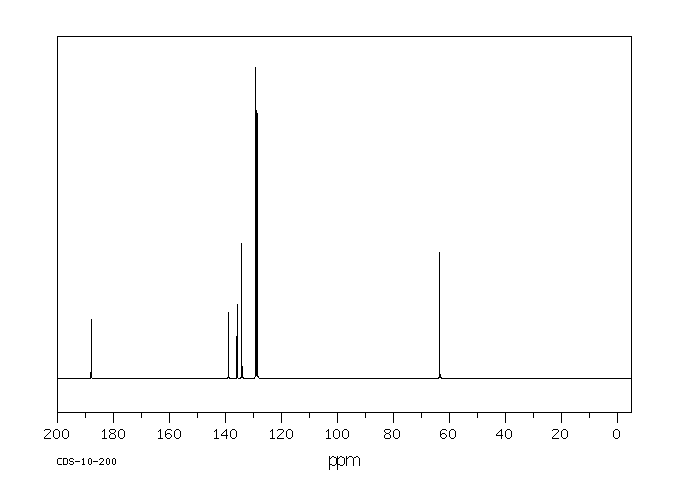
碳谱13CNMR
-
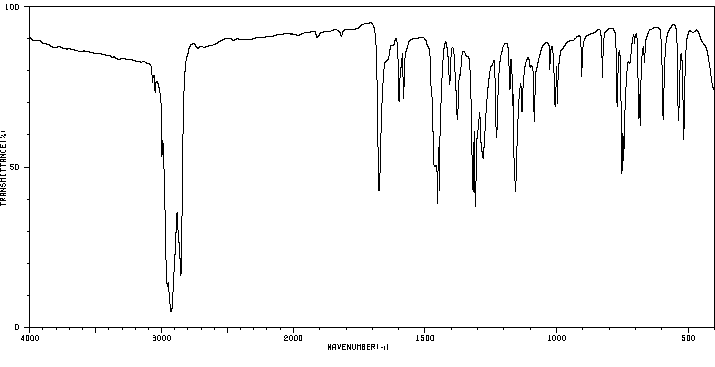
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷