丙二醇甲醚 | 107-98-2
物质功能分类
中文名称
丙二醇甲醚
中文别名
2-羟丙基甲基醚;丙二醇一甲醚;1,2-丙二醇-1-单甲醚;1,2-丙二醇-1-甲乙醚;2-羟丙基・甲基醚;1-甲氧基-2-丙醇;1,2-丙二醇-1-甲醚;1-甲氧基-2-二丙醇;丙二醇单甲醚
英文名称
1-methoxy-2-propanol
英文别名
1-methoxypropan-2-ol;propylene glycol methyl ether;propylene glycol monomethyl ether;PGME
CAS
107-98-2;1320-67-8
化学式
C4H10O2
mdl
——
分子量
90.1222
InChiKey
ARXJGSRGQADJSQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-97 °C
-
沸点:118-119 °C(lit.)
-
密度:0.922 g/mL at 25 °C(lit.)
-
蒸气密度:3.12 (vs air)
-
闪点:93 °F
-
溶解度:水:混溶
-
暴露限值:TLV-TWA 100 ppm (370 mg/m3) (ACGIH); STEL 150 ppm (555 mg/m3) (ACGIH).
-
LogP:0.37 at 20℃
-
物理描述:1-methoxy-2-propanol appears as a colorless liquid. Flash point near 89°F. Less dense than water. Contact irritates skin, eyes and mucous membranes. Prolonged exposure to vapors may cause coughing, shortness of breath, dizziness and intoxication. Vapors heavier than air. Used as a solvent and as an antifreeze agent.
-
颜色/状态:Colorless liquid
-
气味:Weak pleasant odor
-
味道:BITTER TASTE
-
蒸汽密度:3.11 (NTP, 1992) (Relative to Air)
-
蒸汽压力:12.5 mm Hg at 25 °C
-
亨利常数:9.20e-07 atm-m3/mole
-
大气OH速率常数:1.86e-11 cm3/molecule*sec
-
稳定性/保质期:
Volatile liquid.
-
自燃温度:270 °C at 1013 hPa
-
粘度:1.81 mPa-s at 20 °C
-
燃烧热:-556 kcal/mole; -6.18 kcal/g; -11,115 BTU/lb
-
汽化热:46.2 kJ/mol at standard conditions
-
表面张力:27.7 dynes/cm
-
气味阈值:The odor of /propylene glycol monomethyl ether/ could be detected at 10 ppm.
-
折光率:Index of refraction = 1.4034 at 20 °C/D
-
相对蒸发率:0.71 (Butyl acetate = 1)
-
保留指数:673.2;673.4;669;658
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
ADMET
代谢
单次口服给予大鼠一种在糖醇碳上带有放射性标记的丙二醇甲醚。随后48小时内,尿液中出现的放射性以及呼出气体中的放射性被量化并鉴定。大部分给予的丙二醇甲醚被代谢成丙二醇,可能是通过细胞色素P-450依赖的O-去甲基化作用。丙二醇进一步代谢成(14)二氧化碳。
A single oral dose /of propylene glycol monomethyl ether was administered/, radiolabeled in the glycol carbons, to rats. The radioactivity appearing in the urine and expired air over the following 48 hours was quantitiated and identified. Most of the administered propylene glycol monomethyl ether is metabolized to propylene glycol, presumably by cytochrome P-450 dependent O-demethylation. Propylene glycol is further metabolized to (14)carbon dioxide.
来源:Hazardous Substances Data Bank (HSDB)
代谢
与alpha异构体相比,beta-丙二醇单甲醚(PGME)在大鼠体内被氧化为2-甲氧基丙酸,其消除反映了在单次口服剂量大于90 mg/kg的大鼠中药代动力学饱和。大约70到80%的给药beta异构体出现在尿液中,主要是以2-甲氧基丙酸(高达93%)的形式,只有3到4%以beta-PGME葡萄糖苷酸的形式存在。与alpha-PGME不同,其中50到60%的剂量被完全氧化,与beta-PGME相关的放射性标记只有10%以14C-CO2的形式消除。因此,alpha-PGME主要通过肝脏O-去甲基化途径代谢,而beta-PGME异构体则通过醇/醛脱氢酶处理。
In contrast to the alpha-isomer, beta-propylene glycol monomethyl ether (PGME) is oxidized in rats to 2-methoxypropionic acid, and it elimination reflects pharmacokinetic saturation at single oral doses in rats greater than 90 mg/kg. Some 70 to 80% of the administered beta-isomer appeared in urine, primarily as 2-methoxypropionic acid (up to 93%) with only 3 to 4% present as beta-PGME glucuronide. In contrast to alpha-PGME, where 50 to 60 % of the dose was oxidized completely, only 10 % of the radio-label associated with beta-PGME was eliminated as 14C-CO2. Thus, alpha-PGME undergoes hepatic O-demethylation as the principal pathway, whereas the beta-PGME isomer is handled by alcohol/aldehyde dehydrogenase.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Workers exposed to 20-40 ppm PGME for 5 hours had concentrations of 2-8 mg/L PGME appear in their urine, of which 40-60% was in conjugated form (sulfate and glucuronide).
来源:Hazardous Substances Data Bank (HSDB)
代谢
丙二醇甲醚(PM)及其醋酸酯是丙二醇醚类溶剂中使用最广泛的产品。在动物研究中观察到的PM最常见的毒性效应包括镇静、非常轻微的α(2u)-球蛋白介导的肾病(仅限雄性大鼠)以及在较高暴露水平(通常>1000 pPM)时出现的肝肿大。动物研究中的镇静通常在几次暴露于3000 pPM(亚慢性 and 慢性吸入研究中使用的最高浓度)后会因代谢酶的诱导而缓解。来自多种药物动力学和机制研究的数据已经被纳入大鼠和小鼠的PM及其醋酸酯的生理药代动力学(PBPK)模型。已发表的受控暴露和职业生物监测研究也包括在内,用于比较实验室动物和人类之间PM及其醋酸酯的内部剂量学。PM醋酸酯迅速水解为PM,后者进一步代谢为葡萄糖苷酸或硫酸盐结合物(次要途径)或丙二醇(主要途径)。PM醋酸酯的体外半衰期根据组织和物种的不同,从14到36分钟不等。体内半衰期更快,反映了血液和体内组织中酯酶的总贡献,只需几分钟。因此,体内几乎不存在PM醋酸酯,除了潜在的入口刺激外,PM醋酸酯的毒性与其代谢产物PM相关。无论PM的来源是PM还是其醋酸酯,预测大鼠体内PM的血药浓度峰值(Cmax)和药时曲线下面积(AUC)高于人类,特别是在超过当前ACGIH阈限值(TLV)100 pPM的浓度时。这表明,与大鼠在类似的吸入暴露下相比,人类PM的主要系统性效应预期会较轻。
Propylene glycol monomethyl ether (PM), along with its acetate, is the most widely used of the propylene glycol ether family of solvents. The most common toxic effects of PM observed in animal studies include sedation, very slight alpha(2u)-globulin mediated nephropathy (male rats only) and hepatomegally at high exposures (typically > 1000 ppm). Sedation in animal studies usually resolves within a few exposures to 3000 ppm (the highest concentration used in subchronic and chronic inhalation studies) due to the induction of metabolizing enzymes. Data from a variety of pharmacokinetic and mechanistic studies have been incorporated into a PBPK model for PM and its acetate in rats and mice. Published controlled exposure and workplace biomonitoring studies have also been included for comparisons of the internal dosimetry of PM and its acetate between laboratory animals and humans. PM acetate is rapidly hydrolyzed to PM, which is further metabolized to either glucuronide or sulfate conjugates (minor pathways) or propylene glycol (major pathway). In vitro half-lives for PM acetate range from 14 to 36 min depending upon the tissue and species. In vivo half-lives are considerably faster, reflecting the total contributions of esterases in the blood and tissues of the body, and are on the order of just a few minutes. Thus, very little PM acetate is found in vivo and, other than potential portal of entry irritation, the toxicity of PM acetate is related to PM. Regardless of the source for PM (either PM or its acetate), rats were predicted to have a higher Cmax and AUC for PM in blood than humans, especially at concentrations greater than the current ACGIH TLV of 100 ppm. This would indicate that the major systemic effects of PM would be expected to be less severe in humans than rats at comparable inhalation exposures. /propylene glycol monomethyl ether/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:在文献中,1-甲氧基-2-丙醇通常被称为丙二醇单甲醚(PGME)。商业PGME(CAS 130-67-8)通常含有至少98.5%的1-甲氧基-2-丙醇(α-异构体)和最多1.5%的2-甲氧基-1-丙醇(β-异构体)。PGME是一种无色液体,与水和许多有机溶剂混溶。这使得它适用于制造油漆、涂料、染料、油墨、清洁剂和液体肥皂等多种溶剂应用。人类暴露和毒性:PGME α(1-甲氧基-2-丙醇)毒性较低。在男性志愿者中,暴露于100 ppm的PGME后,受试者认为气味太浓无法忍受,但在25分钟内对气味产生了耐受性。在3.5小时的100 ppm暴露期间,6人中有3人出现轻微眼刺激。在250 ppm时,23名受试者在1-7小时的暴露期间,大多数人抱怨眼睛、鼻子或喉咙刺激;几名受试者出现头痛,一人感到恶心。所有暴露均未导致视觉、协调、神经或制动反应测试的变化。临床研究(例如,血液细胞计数、SGOT、SGPT、BUN和完整的尿液分析)在暴露前后的结果显示无影响。体外测试健康个体鼻呼吸上皮原代细胞培养表明,1-甲氧基-2-丙醇诱导编码促炎症细胞因子和介质的基因转录,但很大程度上不翻译这些基因;这表明现有的暴露限值似乎对上呼吸道炎症反应是安全的。动物研究:1-甲氧基-2-丙醇在24小时或48小时暴露后不是豚鼠的皮肤致敏剂,并且在兔子的测试中未引起皮肤刺激。它被分类为对兔子眼睛有轻微刺激性。狗静脉注射的效应是注射部位疼痛、呼吸浅、血压下降、心房心律失常和抽搐导致的死亡。在4小时/天,5天/周,持续2周的10,000 ppm暴露下,大鼠的生长速率降低。兔子的较大皮肤剂量产生中枢神经系统抑制和死亡。在兔子腹部贴上绷带夹子,暴露于10 mL/kg,持续90天(65次剂量)的兔子中也注意到了轻微的肾脏重量增加。在大鼠暴露于0、300、1000或3000 ppm(0、1.09、3.62或10.9 mg/L)的PGME,6小时/天,5天/周,持续13周后,出现了肝细胞增生,但没有退行性变化的证据。在4周的暴露期间,暴露于3000 ppm PGME的雄性大鼠的尿pH值增加。实验室动物的研究表明,PGME不是致癌物;在一项为期2年的研究中,通过吸入暴露于PGME的雄性和雌性小鼠在任何组织中的肿瘤数量都没有增加。PGME已导致发育和生殖毒性。在一项大鼠两代繁殖研究中,通过吸入暴露于3000 ppm的PGME蒸气,每天6小时,每周5天,交配前,以及交配、妊娠和哺乳期间,每天6小时,每周7天,持续两代,P1和P2成鼠表现出毒性,表现为暴露期间和暴露后镇静,平均体重比对照组低21%。这伴随着发情周期延长、生育能力下降、卵巢重量减轻和母鼠卵巢组织学萎缩。从这些母鼠的后代中观察到体重下降、存活率和窝产仔数减少、青春期开始轻微延迟以及F1和F2后代的肝脏和胸腺组织学变化。在1000 ppm时未观察到生殖/新生儿效应。在多项研究中,妊娠期间给小鼠、大鼠和兔子喂食PGME,仅在最高剂量的情况下,在大鼠胎儿中观察到头骨的骨化延迟。PGME在使用TA98、TA100、TA1535、TA 1537和TA1538测试菌株的 Ames沙门氏菌/哺乳动物微体细菌诱变试验(Ames试验)中呈阴性,且对培养中的CHO细胞不具有断裂性。
IDENTIFICATION AND USE: In the literature 1-methoxy-2-propanol is often referred to as propylene glycol monomethyl ether (PGME). Commercial PGME (CAS 130-67-8) typically contains at least 98.5% 1-methoxy-2-propanol (alpha isomer) and only 1.5% of 2-methoxy-1-propanol (beta isomer). PGME is a colorless liquid, miscible with water and many organic solvents. This makes it useful for a wide variety of solvent applications in the manufacture of lacquers, paints, dyes, inks, cleaning agents and liquid soaps. HUMAN EXPOSURE AND TOXICITY: PGME alpha (1-methoxy-2-propanol) has low toxicity. In male volunteers, following exposure to 100 ppm of PGME the subjects felt that the odor was too strong to be tolerated, but odor tolerance developed within 25 min. During a 3.5-hr exposure at 100 ppm, mild eye irritation was noted in 3 of the 6 individuals. At 250 ppm, the majority of the 23 subjects exposed for 1-7 hr complained of eye, nose or throat irritation; several subjects developed headaches, and one was nauseated. None of the exposures resulted in changes in visual, coordination, neurological or brake-reaction tests. Clinical studies (e.g., blood cell count, SGOT, SGPT, BUN and complete urinalysis) completed before and after exposure showed no effects. In vitro testing in primary cell cultures of nasal respiratory epithelia of healthy individuals showed that 1-methoxy-2-propanol induces the transcription of genes encoding proinflammatory cytokines and mediators but largely not translation of those; suggesting the existing exposure limits seem to be safe with respect to inflammatory responses of the upper respiratory tract. ANIMAL STUDIES: 1-Methoxy-2-propanol was not a skin sensitizer in guinea-pigs following 24 or 48 hour exposure and did not cause skin irritation when tested in rabbits. It was classifed as mildly irritating to rabbit eyes. The effects from iv injection in dogs were those of pain at the site of injection, shallow breathing, decreased blood pressure, auricular arrhythmia, and death due to convulsions. A decreased growth rate was seen in rats exposed to 10,000 ppm for 4 hr/day, 5 days/wk for 2 weeks. Large dermal doses in rabbits produced CNS depression and death. A slight increase in kidney weights was also noted in rabbits exposed to 10 mL/kg beneath bandages clipped to abdomens for a 90 day period (65 doses). In rats exposed to 0, 300, 1000, or 3000 ppm (0, 1.09, 3.62, or 10.9 mg/L) of PGME for 6 hr/day, 5 days/wk, for 13 weeks had hepatocellular hypertrophy but were without evidence of degenerative changes. There was an increase in the urinary pH of male rats exposed to 3000 ppm PGME for 4 weeks. Studies in laboratory animals indicate that PGME is not carcinogenic; there were no increases in tumors in any tissue in a 2-year study of male and female mice exposed to PGME via inhalation. PGME has resulted in developmental and reproductive toxicity. In a rat 2-generation reproduction study exposed to 3000 ppm of PGME vapors via inhalation for 6 hours/day, 5 days/week prior to mating, and 6 hours/day, 7 days/week during mating, gestation, and lactation, for 2 generations had toxicity in the P1 and P2 adults, as evidenced by sedation during and after exposure, and mean body weights which were as much as 21% lower than controls. This was accompanied by lengthened estrous cycles, decreased fertility, decreased ovary weights, and histologic ovarian atrophy in maternal rats. In the offspring from these dams, decreased body weights, reduced survival and litter size, slight delays in puberty onset, and histologic changes in liver and thymus in the F1 and F2 offspring were observed. No reproductive/neonatal effects were observed at 1000 ppm. In multiple studies, PGME fed to mice, rats, and rabbits during gestation resulted in delayed ossification of the skull only in the rat fetus; a delayed ossification of the skull, at the highest dose. PGME was negative in the Ames Salmonella/mammalian-microsome bacterial mutagenicity assay (Ames test) using tester strains TA98, TA100, TA1535, TA 1537 and TA1538 and PGME was non-clastogenic to CHO cells in culture.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A4:不能归类为人类致癌物。
A4: Not classifiable as a human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
该物质可以通过吸入其气溶胶或蒸汽、透过皮肤和经口摄入被身体吸收。
The substance can be absorbed into the body by inhalation of its aerosol or vapour, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,吞食,皮肤和/或眼睛接触
inhalation, ingestion, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眼睛、皮肤、鼻子、喉咙刺激;头痛、恶心、眩晕、嗜睡、不协调;呕吐、腹泻
irritation eyes, skin, nose, throat; headache, nausea, dizziness, drowsiness, incoordination; vomiting, diarrhea
来源:The National Institute for Occupational Safety and Health (NIOSH)
吸收、分配和排泄
在反复接触高剂量后,可以通过皮肤吸收达到有毒量。
...Can be absorbed through skin in toxic amount after repeated, high dose exposures.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
F344大鼠对吸入的丙二醇甲醚的总保留率为94%,其中超过96%的值被上呼吸道吸收。在1000 ppm的浓度下,即使循环中的丙二醇甲醚浓度增加,肺消除仍然保持恒定。尽管增加通气对丙二醇甲醚的保留率没有影响,但当通气率加倍时,吸收速率加倍。
Total retention by F344 rats of inhaled propylene glycol methyl ether was 94% with more than 96% of that value being absorbed by the upper respiratory tract. At 1000 ppm, pulmonary elimination remained constant even as circulating propylene glycol methyl ether conc increased. Although increased ventilation had no effect on propylene glycol methyl ether retention, the rate of absorption doubled when the ventilation rate doubled.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
丙二醇甲醚通过人体腹部表皮体外吸收速率为1.17毫克/平方厘米/小时。
Propylene glycol methyl ether was absorbed through human abdominal epidermis in vitro at 1.17 mg/sq cm/hr, ... .
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
经口给药的丙二醇甲醚在大鼠体内表现出快速吸收和剂量依赖性的代谢与消除。将含有90或780毫克(14)C丙二醇甲醚/千克的溶液通过口腔插管给雄性F344大鼠,发现48小时后体内保留了9%的给药剂量,10%到20%出现在尿液中,呼出的二氧化碳占给药剂量的63%。在高剂量下,呼出的二氧化碳占给药的(14)C比例较小,尿液排出并未随着剂量的增加而相应增加。
Orally administered propylene glycol methyl ether exhibits rapid absorption and dose dependent metabolism and elimination in rats. Oral intubation of 90 or 780 mg (14)C propylene glycol methyl ether/kg in tap water to male F344 rats found that 9% of the administered dose was retained by the body at 48 hours after exposure, 10% to 20% appeared in urine, and expired carbon dioxide accounted for 63% of the dose. At the higher dose, expired carbon dioxide accounted for a smaller percentage of administered 14(C), and urinary elimination failed to show a proportionate increase with dose.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 100 ppm (360 mg/m3), STEL: 150 ppm (540 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
危险品标志:T
-
安全说明:S24,S24/25,S26
-
危险类别码:R10
-
WGK Germany:1
-
海关编码:2909499000
-
危险品运输编号:UN 3092 3/PG 3
-
危险类别:3
-
RTECS号:UB7700000
-
包装等级:III
-
危险性防范说明:P210,P233,P240,P241,P242,P243,P260,P261,P271,P280,P303+P361+P353,P304,P304+P340,P312,P340,P370+P378,P403,P403+P233,P403+P235,P405,P501
-
危险性描述:H225,H336
SDS
制备方法与用途
简介
丙二醇醚对人体的毒性低于乙二醇醚类产品,属于低毒醚类。其中,丙二醇甲醚具有轻微的醚味,无强烈刺激性气味,这使其应用更为广泛且安全。丙二醇甲醚是一种环保型有机溶剂,在化工生产中用途极广。
溶解性丙二醇甲醚溶解性强、毒性低,并能与水及多种有机溶剂混溶;偶联性能良好,表面张力低,粘度较低,且蒸发速率高。
回收方法在涂料制备、造纸化学品生产、纸张表面处理等化工过程中会大量产生丙二醇甲醚的废弃液。回收丙二醇甲醚的研究意义重大,不仅具有显著的经济效益,还利于环境保护。其沸点为118~119℃,接近于苯和甲苯的沸点,并可与水互溶,在实验中如何有效分离是一个难点。郗伟等人采用共沸精馏技术,通过在废弃液中加入适量无水乙醇作为共沸剂,在连续化、产量稳定的条件下,成功分离出高纯度目标产物。具体步骤如下:
- 精馏塔按间歇生产工艺控制,将原料液一次性加入塔釜。
- 缓慢加热,并保持蒸汽压力在0.3~0.4MPa之间。
- 低沸点馏分开始从塔顶蒸出后进行全回流处理,持续时间设定为36~60分钟。
- 待塔顶温度稳定后开启塔顶馏出物阀门,控制回流比在1:1至1:0.5之间,并记录温度。
- 塔顶温度低于110℃时按轻组分蒸出;110~115℃为可疑组分阶段,需分两阶段取样分析确认;大于115℃的馏分为正品,每增加1℃采样一组,最终确定塔顶温度。
丙二醇甲醚是一种液体,熔点-97℃,沸点118~119℃,折射率20D为1.4030,密度0.9220,闪点33℃。它可以溶解于醚、氯仿等有机溶剂中。
用途丙二醇甲醚作为溶剂、分散剂或稀释剂广泛应用于涂料、油墨、印染、农药、纤维素及丙烯酸酯等工业;也可用作燃料抗冻剂、清洗剂、萃取剂和有色金属选矿剂;还可作为有机合成原料。
生产方法通过1,2-环氧丙烷与甲醇在催化剂存在下反应,再进行粗馏精馏过程制得丙二醇甲醚。
类别易燃液体
毒性分级中毒
急性毒性口服 - 大鼠 LD50: 3739毫克/公斤;小鼠 LD50: 11700毫克/公斤
刺激数据皮肤 - 兔子,500毫克 轻度刺激 眼睛 - 兔子,500毫克/24小时 轻度刺激
爆炸物危险特性与空气混合可燃爆炸
可燃性危险特性易燃,在火场会产生辛辣刺激烟雾
储运特性应贮存在阴凉、干燥通风的场所,避免阳光直射。与氧化剂分开存放。
灭火剂 职业标准时间加权平均容许浓度(TWA)360毫克/立方米;短时间接触容许浓度(STEL)1080毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2-二甲氧基丙烷 1,2-dimethoxypropane 7778-85-0 C5H12O2 104.149 (甲氧基甲基)环氧乙烷 glycidyl methyl ether 930-37-0 C4H8O2 88.1063 1,2-丙二醇 1,2-Propanediol 57-55-6 C3H8O2 76.0953 4-甲基-1,3-二氧戊环 4-methyl-1,3-dioxolane 1072-47-5 C4H8O2 88.1063 —— methyloxirane 16033-71-9 C3H6O 58.08 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (S)-(+)-1-甲氧基-2-丙醇 (S)-1-Methoxy-propan-2-ol 26550-55-0 C4H10O2 90.1222 (R)-(-)-1-甲氧基-2-丙醇 (R)-1-methoxy-2-propanol 4984-22-9 C4H10O2 90.1222 —— methyloxirane 16033-71-9 C3H6O 58.08
反应信息
-
作为反应物:描述:丙二醇甲醚 在 盐酸 、 2,2,6,6-四甲基哌啶氧化物 、 sodium nitrite 作用下, 以 二氯甲烷 、 水 为溶剂, 20.0 ℃ 、101.33 kPa 条件下, 反应 31.0h, 以89%的产率得到1-甲氧基-2-丙酮参考文献:名称:TEMPO / HCl / NaNO2催化剂:一种无过渡金属的方法,可在温和条件下有效地将酒精好氧氧化为醛和酮。摘要:已发现盐酸是一种非常便宜且易于获得的无机酸,可与NaNO(2)/ TEMPO精巧地合作,催化分子氧驱动的多种醇底物氧化成相应的醛和酮。这种无过渡金属的催化氧化转化是新颖的,代表了相应的羰基化合物在金属催化的醇的有氧氧化中的有趣替代途径。当在室温,大气压下于室温下进行时,该反应对所需产物具有高度选择性。值得注意的是,非常便宜的NaNO(2)和HCl与TEMPO结合使用,可以在环境温度下对空气中的酒精进行高度选择性的需氧氧化,从而使该反应在操作上和经济上都非常有吸引力。介绍并讨论了借助电喷雾电离质谱(ESI-MS)进行的机理研究的结果。通过ESI-MS在氧化还原循环中观察到TEMPO,TEMPOH和TEMPO(+)。基于这些观察,提出了一种机制,可以提供对新近发展的好氧醇氧化的见解。DOI:10.1002/chem.200701818
-
作为产物:描述:1-甲氧基-2-丙酮 在
氢气 作用下, 以 二乙二醇二甲醚 、 水 为溶剂, 30.0 ℃ 、98.06 kPa 条件下, 反应 20.0h, 生成 丙二醇甲醚 参考文献:名称:酮在阳离子铑配合物的均相催化加氢中的反应性摘要:已经在大气压力下于30°C和氢气下研究了带有阳离子铑络合物的几种酮的催化加氢反应。反应速率在很大程度上取决于酮和膦配体的结构,其中三乙基膦在所使用的配体中具有最高的活性。发现酮中的吸电子取代基可提高其在烷基和芳基酮之间的反应性;前者酮的反应速度通常更快。苄基甲基酮在芳基酮中显示出很高的反应性,表明苯基可能处于与铑原子相互作用的位置,从而增强了反应性。首先将不饱和酮催化氢化以产生饱和酮,然后将其进一步氢化成相应的饱和醇。即使在连续氢化中,即使在不饱和酮中的烯键的氢化速率比相应的饱和酮的羰基的氢化速率小得多时,在该氢化中也未观察到痕量的不饱和醇。结合酮的配位和氢化机理讨论了酮的反应性。DOI:10.1039/p19810002650 -
作为试剂:描述:参考文献:名称:JP5672788摘要:公开号:
文献信息
-
[EN] PYRROLOTRIAZINES AS ALK AND JAK2 INHIBITORS<br/>[FR] PYRROLOTRIAZINES EN TANT QU'INHIBITEURS D'ALK ET DE JAK2申请人:CEPHALON INC公开号:WO2010071885A1公开(公告)日:2010-06-24The present invention provides a compound of formula (I) or a salt form thereof, wherein Q1, Q2, Q3, and Q4 are as defined herein. The compound of formula (I) has ALK and/or JAK2 inhibitory activity, and may be used to treat proliferative disorders.本发明提供了一种式(I)的化合物或其盐形式,其中Q1、Q2、Q3和Q4如本文所定义。式(I)的化合物具有ALK和/或JAK2抑制活性,并可用于治疗增殖性疾病。
-
[EN] BENZIMIDAZOLE DERIVATIVES AS BROMODOMAIN INHIBITORS<br/>[FR] DÉRIVÉS DE BENZIMIDAZOLE COMME INHIBITEURS DES BROMODOMAINES申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2016146738A1公开(公告)日:2016-09-22Compounds of formula (I) and salts thereof: wherein R1, R2, R3, R4 are defined herein. Compounds of formula (I) and salts thereof have been found to inhibit the binding of the BET family of bromodomain proteins to, for example, acetylated lysine residues and thus may have use in therapy, for example in the treatment of autoimmune and inflammatory diseases, such as rheumatoid arthritis; and cancers.
-
Electrochemical Oxidative Carbon‐Atom Difunctionalization: Towards Multisubstituted Imino Sulfide Ethers作者:Zhipeng Guan、Shuxiang Zhu、Siyuan Wang、Huamin Wang、Siyuan Wang、Xingxing Zhong、Faxiang Bu、Hengjiang Cong、Aiwen LeiDOI:10.1002/anie.202011329日期:2021.1.18of functional molecules and natural products. Nonetheless, the synthesis of imino sulfide ethers, containing an N(sp2)=C(sp2)−O/S fragment, still remains a challenge because of its sensitivity to acid. Developed here in is an unprecedented electrochemical oxidative carbon‐atom difunctionalization of isocyanides, providing a series of novel multisubstituted imino sulfide ethers. Under metal‐free and
-
[EN] FUSED RING COMPOUNDS AND USE THEREOF<br/>[FR] COMPOSÉS CYCLIQUES CONDENSÉS ET UTILISATION DE CEUX-CI申请人:TAKEDA PHARMACEUTICAL公开号:WO2009125873A1公开(公告)日:2009-10-15The present invention aims to provide a glucokinase activator useful as a pharmaceutical agent such as an agent for the prophylaxis or treatment of diabetes, obesity and the like. The present invention provides a glucokinase activator containing a compound represented by the formula (I): wherein each symbol is defined in the specification, or a salt thereof or a prodrug thereof.
-
Synthesis of 13-alkyl-gon-4-ones申请人:Smith; Herchel公开号:US03959322A1公开(公告)日:1976-05-25The preparation of 13-methylgon-4-enes and novel 13-polycarbonalkylgon-4-enes by a new total synthesis is described. 13-Alkylgon-4-enes having progestational, anabolic and androgenic activities are prepared by forming a tetracylic gonane structure unsaturated in the 1,3,5(10),9(11) and 14-positions, selectively reducing in the B- and C-rings, and converting the aromatic A-ring compounds so-produced to gon-4-enes by Birch reduction and hydrolysis.描述了通过新的全合成方法制备13-甲基孕-4-烯和新型13-聚碳烷基孕-4-烯。通过形成在1,3,5(10),9(11)和14-位置不饱和的四环孕烷结构,选择性地在B和C环中还原,并将所产生的芳香A环化合物转化为孕-4-烯,制备具有孕激素、合成激素和雄激素活性的13-烷基孕-4-烯。
表征谱图
-
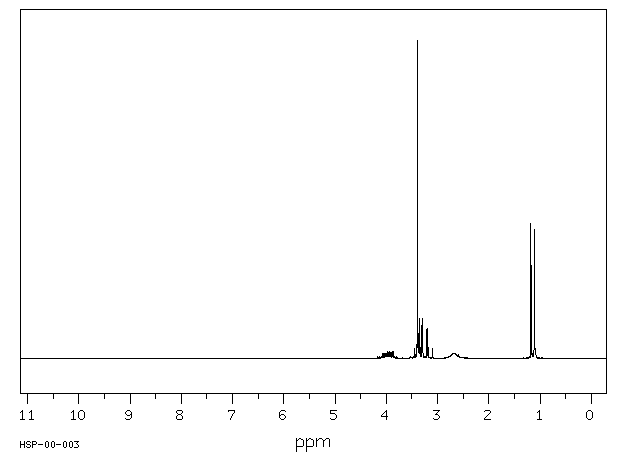
氢谱1HNMR
-
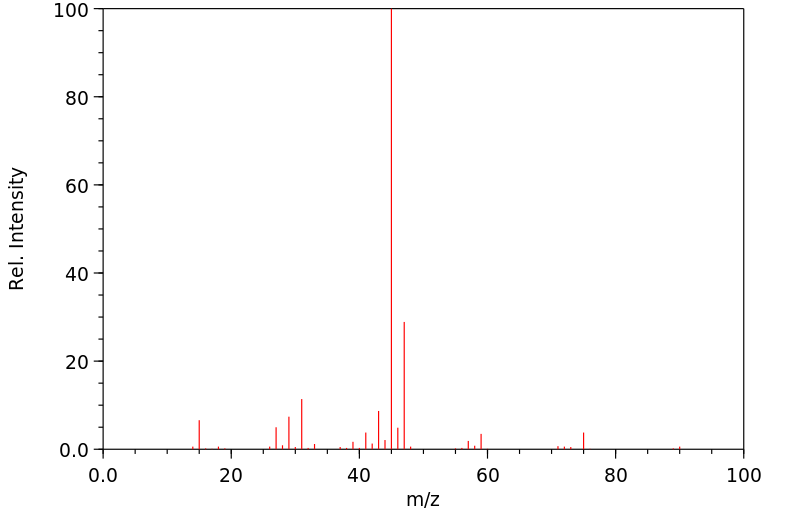
质谱MS
-
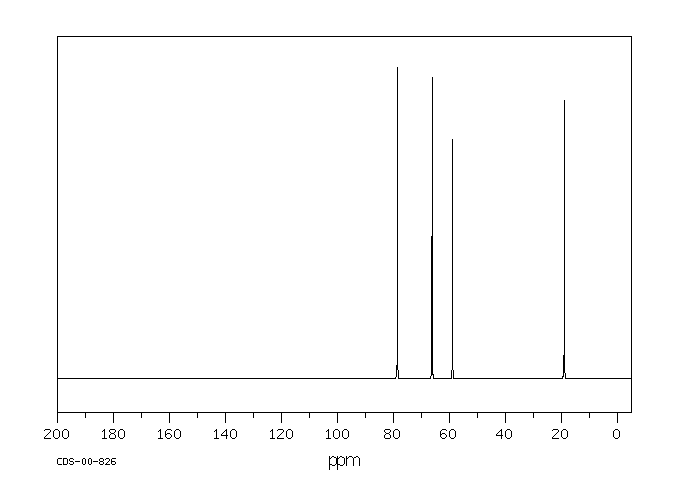
碳谱13CNMR
-
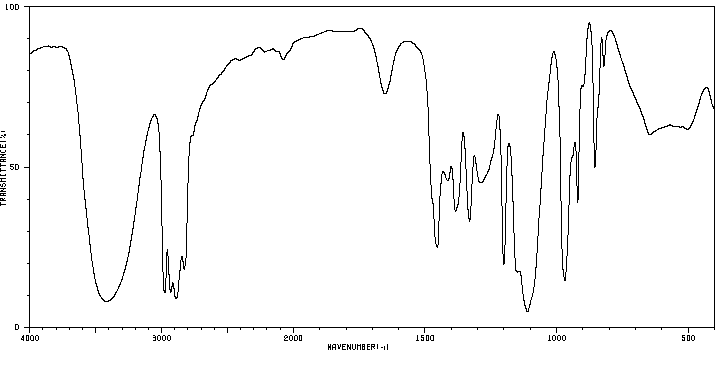
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷