3,5-二甲氧基苯甲酸甲酯 | 2150-37-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:42-43 °C (lit.)
-
沸点:298 °C (lit.)
-
密度:1.2166 (rough estimate)
-
闪点:>230 °F
-
溶解度:在甲醇中几乎透明
-
LogP:1.850 (est)
-
保留指数:1541;1510
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2918990090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:储存地点应远离氧化剂,并确保容器密封良好,存放在密闭容器中。请将其置于阴凉、干燥处。
SDS
: 3,5-二甲氧基苯甲酸甲酯
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C10H12O4
分子式
: 196.2 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 42 - 43 °C - lit.
f) 起始沸点和沸程
298 °C - lit.
g) 闪点
> 113.00 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
化学性质:白色或类白色的结晶粉末,熔点为41-43℃。
用途:广泛用作医药中间体,并应用于有机合成。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二甲氧基苯甲酸 3,5-dimethoxybenzoic acid 1132-21-4 C9H10O4 182.176 3-羟基-5-甲氧基苯甲酸 3-Hydroxy-5-methoxy-benzoesaeure 19520-75-3 C8H8O4 168.149 3,5-二羟基苯甲酸甲酯 1-carbomethoxy-3,5-dihydroxybenzene 2150-44-9 C8H8O4 168.149 丁香酸甲酯 methyl syringate 884-35-5 C10H12O5 212.202 丁香酸 Syringic acid 530-57-4 C9H10O5 198.175 3,5-二羟基苯甲酸 3,5-Dihydroxybenzoic acid 99-10-5 C7H6O4 154.122 3,5-二甲氧基苯甲醛 3,5-dimethoxybenzaldehdye 7311-34-4 C9H10O3 166.177 3,5-二甲氧基苄醇 3,5-dimethoxybenzyl alcohol 705-76-0 C9H12O3 168.192 —— methyl 4-acetoxy-3,5-dimethoxybenzoate 890-47-1 C12H14O6 254.24 间羟基苯甲酸 3-Carboxyphenol 99-06-9 C7H6O3 138.123 苯甲酸,2-溴-3,5-二甲氧基-,甲基酯 methyl 2-bromo-3,5-dimethoxybenzoate 19491-18-0 C10H11BrO4 275.099 没食子酸 3,4,5-trihydroxybenzoic acid 149-91-7 C7H6O5 170.122 3,5-二甲氧基苯甲酰氯 3,5-dimethoxybenzoyl chloride 17213-57-9 C9H9ClO3 200.622 原儿茶酸 3,4-Dihydroxybenzoic acid 99-50-3 C7H6O4 154.122 2,5-二羟基苯甲酸 2,5-dihydroxybenzoic acid. 490-79-9 C7H6O4 154.122 苯甲酸,二羟基- 2,3-Dihydroxybenzoic acid 303-38-8 C7H6O4 154.122 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,5-二乙氧基基苯甲酸甲酯 ethyl 3,5-dimethoxybenzoate 17275-82-0 C11H14O4 210.23 3,5-二甲氧基苯甲酸 3,5-dimethoxybenzoic acid 1132-21-4 C9H10O4 182.176 1,3-二甲氧基-5-(甲氧基甲基)苯 3,5-dimethoxybenzyl methyl ether 73569-69-4 C10H14O3 182.219 —— methyl 3,5-dimethoxy-4-aminobenzoate 56066-25-2 C10H13NO4 211.218 3,5-二甲氧基苯甲醛 3,5-dimethoxybenzaldehdye 7311-34-4 C9H10O3 166.177 3,5-二甲氧基苄醇 3,5-dimethoxybenzyl alcohol 705-76-0 C9H12O3 168.192 3,5-二甲氧基苄基乙酸酯 3,5-dimethoxybenzyl acetate 38513-65-4 C11H14O4 210.23 —— 2-Hydroxy-3,5-dimethoxy-benzoesaeure-methylester 99187-06-1 C10H12O5 212.202 —— methyl 3,5-dimethoxy-4-isopropylbenzoate 344396-17-4 C13H18O4 238.284 3,5-二甲氧基水杨酸 3,5-dimethoxysalicylic acid 61637-60-3 C9H10O5 198.175 —— methyl 2-formyl-3-hydroxy-5-methoxybenzoate 65976-78-5 C10H10O5 210.186 苯甲酸,2-溴-3,5-二甲氧基-,甲基酯 methyl 2-bromo-3,5-dimethoxybenzoate 19491-18-0 C10H11BrO4 275.099 —— methyl 2-iodo-3,5-dimethoxybenzoate 114605-46-8 C10H11IO4 322.099 —— methyl 2-chloro-3,5-dimethoxybenzoate 51903-93-6 C10H11ClO4 230.648 2-甲酰基-3,5-二甲氧基苯甲酸甲酯 methyl 2-formyl-3,5-dimethoxybenzoate 52344-93-1 C11H12O5 224.213 2-氟-3,5-二甲氧基苯甲酸甲酯 methyl 2-fluoro-3,5-dimethoxybenzoate 651734-58-6 C10H11FO4 214.193 3,5-二甲氧基苯甲酰胺 3,5-dimethoxybenzamide 17213-58-0 C9H11NO3 181.191 3,5-二甲氧基苯甲酰氯 3,5-dimethoxybenzoyl chloride 17213-57-9 C9H9ClO3 200.622 —— 4-isopropyl-3,5-dimethoxybenzoic acid 55703-81-6 C12H16O4 224.257 —— 2,4-dimethoxy-6-methoxymethyltoluene 114972-97-3 C11H16O3 196.246 —— 2-Brom-3,5-dimethoxy-benzoesaeure 17275-86-4 C9H9BrO4 261.072 —— methyl 2,6-dibromo-3,5-dimethoxybenzoate —— C10H10Br2O4 353.995 2-(3,5-二甲氧基苯基)丙-2-醇 3,5-dimethoxy-α,α-dimethyl benzyl alcohol 39507-96-5 C11H16O3 196.246 2,6-二氟-3,5-二甲氧基苯甲酸甲酯 methyl 2,6-difluoro-3,5-dimethoxybenzoate 651734-55-3 C10H10F2O4 232.184 3,5-二甲氧基甲苯 1,3-dimethoxy-5-methylbenzene 4179-19-5 C9H12O2 152.193 —— 4,6-dimethoxy-phthalide 58545-97-4 C10H10O4 194.187 2-碘-3,5-二甲氧基苯甲酸 2-iodo-3,5-dimethoxybenzoic acid 124481-00-1 C9H9IO4 308.073 —— 2,4-dimethoxy-6-(methoxymethyl)benzaldehyde 114973-03-4 C11H14O4 210.23 —— 1-(3,5-dimethoxyphenyl)propan-1-one 41497-31-8 C11H14O3 194.23 —— 3,5-dimethoxysalicylic alcohol 946408-35-1 C9H12O4 184.192 3,5-二甲氧基苯甲酰肼 3,5-dimethoxy-benzoic acid hydrazide 51707-38-1 C9H12N2O3 196.206 —— 2-formyl-5-methoxy-3-methoxymethoxybenzoic acid methyl ester —— C12H14O6 254.24 2,6-二氟-3,5-二甲氧基苯甲酸 2,6-difluoro-3,5-dimethoxybenzoic acid 651734-56-4 C9H8F2O4 218.157 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:通过改进的 Ullmann-Ziegler 交叉偶联合成大麻酚摘要:大麻酚是一种药学上令人感兴趣的大麻成分,它是通过改进的 Ullmann-Ziegler 交叉偶联制备的。使用容易获得的起始材料,这种收敛方法可以轻松获得各种大麻酚衍生物。DOI:10.1055/s-0033-1338428
-
作为产物:描述:参考文献:名称:A convenient synthesis of14C-labelled resveratrol摘要:白藜芦醇(反式-3,4',5-三羟基芪)是一种天然植物抗毒素和多酚物质,存在于葡萄和各种植物中。它在预防癌症、炎症和血小板聚集等方面显示出显著的有益生物活性。本文报道了以14C-甲酸(与14C-甲酸钠交换)和3,5-二羟基苯甲酸为原料合成[β-14C]-反式白藜芦醇的方法。[14C-甲酰基]-4-甲氧基苯甲醛和二乙基3,5-二甲氧基苄基膦酸酯通过Wittig-Horner反应得到反式-3,4'-5-[β-14C]-三甲氧基芪。最终产物通过反式-3,4'-5-[β-14C]-三甲氧基芪的脱甲基化获得,并通过TLC和UV光谱鉴定。该方法合成的14C-白藜芦醇比活性为40.8 µCi/mmol,化学产率为15.3%,放射化学产率为12.5%。版权所有 © 2004 John Wiley & Sons, Ltd.DOI:10.1002/jlcr.806
文献信息
-
Modified approach for preparing (E)-stilbenes related to resveratrol, and evaluation of their potential immunobiological effects作者:Jan Šmidrkal、Juraj Harmatha、Miloš Buděšínský、Karel Vokáč、Zdeněk Zídek、Eva Kmoníčková、Roman Merkl、Vladimír FilipDOI:10.1135/cccc2009531日期:——
Resveratrol and closely related stilbenoids belong to the most intensively studied biologically active compounds. This interest evoked several attempts to prepare such compounds in a convenient synthetic way. Our approach allowed obtaining largely methoxystilbenes, formed as
E -isomers only (using Wittig–Horner synthesis as the key step), which were further demethylated by boron tribromide. The hydroxymethoxystilbenes (e.g. pterostilbene) were prepared using isopropyl protection, later selectively deprotected by boron trichloride. The method is suitable for preparing such compounds in a large amount. Effects of the obtained stilbene derivatives on immunobiological responses triggered by lipopolysacharide and interferon-γ were tested under in vitro conditions. Namely production of nitric oxide (NO) was investigated, and relation between the molecular structure and immunobiological activity was assessed. -
一种氨基嘧啶化合物申请人:广东中科药物研究有限公司公开号:CN109988156B公开(公告)日:2021-12-28本发明公开了一种氨基嘧啶化合物。该类化合物的结构通式如式I所示。所述式I中,R1=碳原子总数为1‑6的烷基、碳原子总数为3‑6的环烷基、卤素取代的碳原子总数为1‑6的烷基或卤素取代的碳原子总数为3‑6的环烷基;R2=碳原子总数为1‑6的烷氧基、卤素取代的碳原子总数为1‑6的烷氧基、碳原子总数为3‑6的环烷基或卤素取代的碳原子总数为3‑6的环烷基;X=F、Br或Cl。该类化合物具有显著的抑菌药效及良好的成药性。
-
Synthesis of [6,6,m]-Tricyclic Compounds via [4+2] Cycloaddition with Au or Cu Catalyst作者:Chang Ho Oh、Juyeon Kang、Seunghwan Ham、Chaehyeon SeongDOI:10.1055/a-1479-6005日期:2021.6We synthesized [6,6,6]- and [6,6,7]-tricyclic compounds via intramolecular [4+2] cycloaddition by gold or copper catalysts. Substrates for cyclization were prepared by coupling reactions between eight types of diyne and four types of aromatic moieties. We have successfully synthesized eleven tricyclic compounds.
-
Asymmetric Total Syntheses of Cochliomycin A and Zeaenol作者:Nandan Jana、Samik NandaDOI:10.1002/ejoc.201200241日期:2012.8The first asymmetric total syntheses of two resorcylic acid lactones (RALs) – cochliomycin A and zeaenol – have been achieved in a divergent way. The main highlight of our strategy involves successful application of stereoselecive Keck allylation and Julia–Kocienski olefination to access an advanced intermediate, by starting from L-tartaric acid as a chiral pool compound. This intermediate is coupled
-
Inhibitory effect of a novel resveratrol derivative on nitric oxide production in lipopolysaccharide-activated microglia作者:Meng、Chen、Yang、Wang、Wu, Chun Fu、WangDOI:10.1691/ph.2008.8553日期:——Excessive nitric oxide (NO) production by activated microglial cells has been implicated in various neurodegenerative diseases. In the present study, we found that a new resveratrol derivative, (E)-5-(3-nitrostyryl)benzene-1,3-diol (RV06), has a more potential inhibitory effect on the production of NO in LPS-activated N9 microglial cells, and the result was confirmed on primary rat microglial cells. Further studies showed that RV06 inhibited LPS-induced iNOS expression in N9 microglial cells, with no activity on direct scavenging nitric oxide radical in a cell-free environment. The results suggest that RV06 might be a potential anti-inflammatory agent or leading compound which can inhibit inflammatory responses of microglia.
表征谱图
-
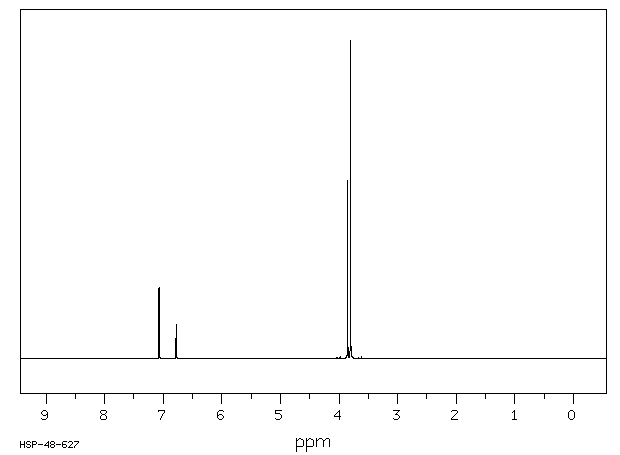
氢谱1HNMR
-
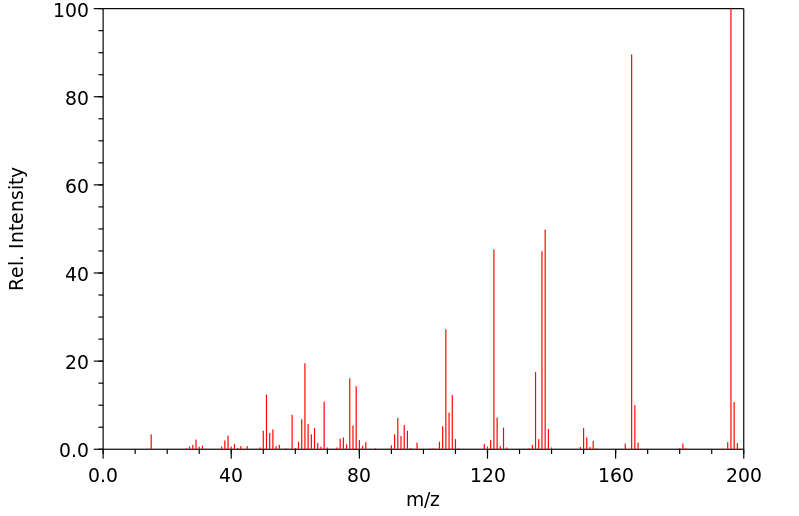
质谱MS
-
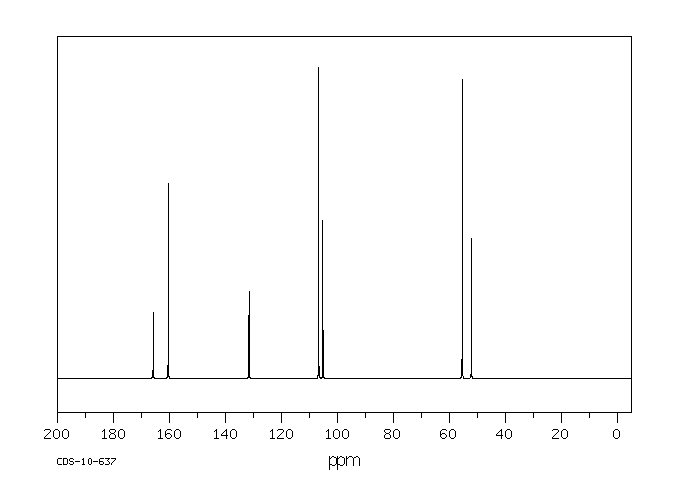
碳谱13CNMR
-
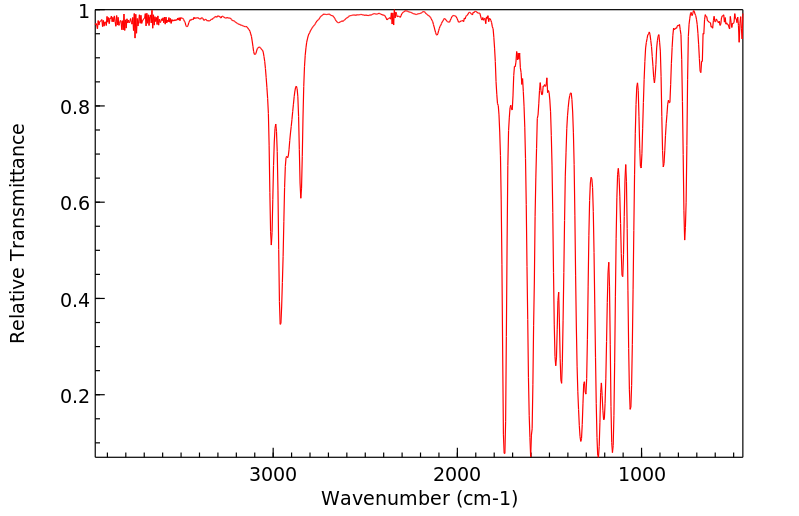
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息