3,5-二甲氧基苯甲酰胺 | 17213-58-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:145-148 °C (lit.)
-
沸点:314.29°C (rough estimate)
-
密度:1.2375 (rough estimate)
-
稳定性/保质期:
常温常压下稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:61.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
安全说明:S24/25,S26
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:2924299090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温下应密闭避光保存,并放置于通风干燥处。
SDS
: 3,5-二甲氧基苯甲酰胺
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H11NO3
分子式
: 181.19 g/mol
分子量
组分 浓度或浓度范围
3,5-Dimethoxybenzamide
-
CAS 号 17213-58-0
EC-编号 241-257-0
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 145 - 148 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 0.999
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
3,5-二甲氧基苯甲酰胺可作为医药合成中间体。
制备3,5-二甲氧基苯甲酰胺可通过以下步骤制备:
-
在500 mL圆底烧瓶中加入3,5-二甲氧基苯甲酸(10.0 g,55 mmol),然后加入50 mL氯化亚砜溶解。加热至90℃回流2小时(TLC跟踪)。反应结束后,在60℃减压蒸干氯化亚砜,得到棕黄色油状物质12.11 g,无需纯化直接用于下一步。
-
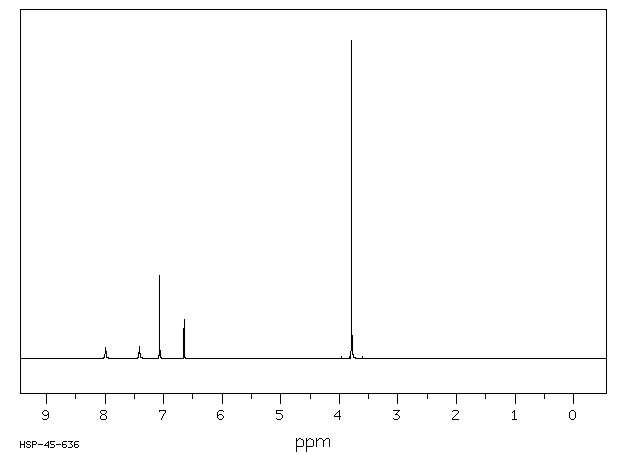
在另一500 mL圆底烧瓶中加入50 mL氨水,并冰浴冷却20分钟。将步骤1的产物(11.0 g,55 mmol)溶于50 mL乙腈,缓慢滴加到三口烧瓶中。期间会产生大量白色固体。反应在室温下继续进行1.5小时(TLC跟踪)。反应结束后,转移至圆底烧瓶中,在50℃减压蒸干乙腈。用水洗涤所得的白色固体,抽滤并真空干燥得到3,5-二甲氧基苯甲酰胺137.8 g,产率为78.4%;1H NMR(400 MHz,CDCl₃):δ 7.03 (d, 2H),6.63 (t, 1H),3.80 (s, 6H)。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二甲氧基苯甲酰肼 3,5-dimethoxy-benzoic acid hydrazide 51707-38-1 C9H12N2O3 196.206 3,5-二甲氧基苯甲酰叠氮化物 3,5-dimethoxybenzoyl azide 75996-26-8 C9H9N3O3 207.189 3,5-二甲氧基苯甲酸 3,5-dimethoxybenzoic acid 1132-21-4 C9H10O4 182.176 3,5-二甲氧基苯甲酰氯 3,5-dimethoxybenzoyl chloride 17213-57-9 C9H9ClO3 200.622 3,5-二甲氧基苯甲酸甲酯 methyl 3,5-dimethoxybenzoate 2150-37-0 C10H12O4 196.203 3,5-二乙氧基基苯甲酸甲酯 ethyl 3,5-dimethoxybenzoate 17275-82-0 C11H14O4 210.23 3,5-二甲氧基苯腈 3,5-dimethoxy-benzonitrile 19179-31-8 C9H9NO2 163.176 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,5-dimethoxy-N-methylbenzamide 74826-21-4 C10H13NO3 195.218 —— 3,5-dimethoxybenzoyl isocyanate 1061639-77-7 C10H9NO4 207.186 —— 3,5-dimethoxy-N,N-dimethylbenzamide 116114-87-5 C11H15NO3 209.245 —— N-benzyl-3,5-dimethoxybenzamide —— C16H17NO3 271.316 3,5-二甲氧基苄胺 3,5-dimethoxybenzylamine 34967-24-3 C9H13NO2 167.208 —— N-acetyl-3,5-dimethoxybenzamide 73823-21-9 C11H13NO4 223.229 3,5-二甲氧基-N-苯基苯甲酰胺 3,5-dimethoxy-N-phenylbenzamide 87282-04-0 C15H15NO3 257.289 3,5-二甲氧基苯甲酸 3,5-dimethoxybenzoic acid 1132-21-4 C9H10O4 182.176 —— N-cyclohexyl-3,5-dimethoxybenzamide 349108-56-1 C15H21NO3 263.337 —— 3,5-dimethoxy-N-(4-methylphenyl)benzamide 328969-54-6 C16H17NO3 271.316 3,5-二甲氧基硫代苯甲酰胺 3,5-dimethoxybenzothioamide 114980-23-3 C9H11NO2S 197.258 3,5-二甲氧基苯乙酮 1-(3,5-dimethoxyphenyl)ethan-1-one 39151-19-4 C10H12O3 180.203 —— N-(4-bromophenyl)-3,5-dimethoxybenzamide 328975-22-0 C15H14BrNO3 336.185 —— N-(4-chlorophenyl)-3,5-dimethoxybenzamide 331828-14-9 C15H14ClNO3 291.734 —— 3,5-dimethoxy-N-(thiophen-2-ylmethyl)benzamide —— C14H15NO3S 277.344 —— 1-(3,5-dimethoxyphenyl)propan-1-one 41497-31-8 C11H14O3 194.23 —— Dideuterio-(3,5-dimethoxyphenyl)methanamine 1208988-65-1 C9H13NO2 169.192 3,5-二甲氧基苄醇 3,5-dimethoxybenzyl alcohol 705-76-0 C9H12O3 168.192 —— 1-(3,5-dimethoxyphenyl)-2-methylpropan-1-one 73109-77-0 C12H16O3 208.257 3,5-二甲氧基苯腈 3,5-dimethoxy-benzonitrile 19179-31-8 C9H9NO2 163.176 —— 1-(3,5-dimethoxyphenyl)butan-1-one 39911-73-4 C12H16O3 208.257 1-(3,5-二甲氧基苯基)戊-1-酮 1-(3,5-dimethoxyphenyl)pentan-1-one 5333-29-9 C13H18O3 222.284 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Durrani, Aziz A.; Tyman, John H. P., Journal of the Chemical Society. Perkin transactions I, 1980, p. 1658 - 1666摘要:DOI:
-
作为产物:描述:参考文献:名称:使用过硼酸钠进一步氧化官能团摘要:在乙酸过硼酸钠是芳族醛氧化成羧酸的有效试剂,iodoarenes至(二乙酰氧基碘)芳烃,吖嗪类,以-oxides,以及各种类型的硫杂环到,-dioxides。腈在乙酸中不受试剂的影响,但是当使用甲醇水溶液作为溶剂时,腈会平稳地水合为酰胺。DOI:10.1016/s0040-4020(01)81008-5
文献信息
-
Transition metal-free catalytic reduction of primary amides using an abnormal NHC based potassium complex: integrating nucleophilicity with Lewis acidic activation作者:Mrinal Bhunia、Sumeet Ranjan Sahoo、Arpan Das、Jasimuddin Ahmed、Sreejyothi P.、Swadhin K. MandalDOI:10.1039/c9sc05953a日期:——potassium complex was used as a transition metal-free catalyst for reduction of primary amides to corresponding primary amines under ambient conditions. Only 2 mol% loading of the catalyst exhibits a broad substrate scope including aromatic, aliphatic and heterocyclic primary amides with excellent functional group tolerance. This method was applicable for reduction of chiral amides and utilized for the synthesis
-
Amyl nitrite-mediated conversion of aromatic and heteroaromatic primary amides to carboxylic acids作者:Garrett T. Potter、Gordon C. Jayson、Gavin J. Miller、John M. GardinerDOI:10.1016/j.tetlet.2015.05.054日期:2015.9A series of aromatic and heteroaromatic primary amides were converted directly to carboxylic acids by heating with amyl nitrite in acetic acid. Most conversions proceeded to give reasonable to excellent yields on a range of substrates containing various functional groups. This reagent system is thus applicable for the direct hydrolysis of a range of different primary carboxamides. The reaction with
-
Design, synthesis and Structure–activity relationship studies of new thiazole-based free fatty acid receptor 1 agonists for the treatment of type 2 diabetes作者:Zheng Li、Qianqian Qiu、Xue Xu、Xuekun Wang、Lei Jiao、Xin Su、Miaobo Pan、Wenlong Huang、Hai QianDOI:10.1016/j.ejmech.2016.02.040日期:2016.5The free fatty acid receptor 1 (FFA1/GPR40) has attracted interest as a novel target for the treatment of type 2 diabetes. Several series of FFA1 agonists including TAK-875, the most advanced compound terminated in phase III studies due to concerns about liver toxicity, have been hampered by relatively high molecular weight and lipophilicity. Aiming to develop potent FFA1 agonists with low risk of游离脂肪酸受体1(FFA1 / GPR40)作为治疗2型糖尿病的新靶标已引起人们的关注。相对较高的分子量和亲脂性阻碍了包括FAK1激动剂在内的数个系列的FFA1激动剂(由于对肝毒性的担忧而在III期研究中终止的最先进的化合物)。为了通过降低亲脂性来开发具有低肝毒性风险的有效FFA1激动剂,TAK-875的中间苯基被11个极性五元杂芳族化合物所取代。随后,对SAR的系统探索和分子建模的应用导致了化合物44的鉴定,它是一种出色的FFA1激动剂,在正常和2型糖尿病小鼠中均具有强大的降血糖作用,即使在两倍摩尔的TAK-875剂量下也具有低血糖风险和肝毒性。同时,指出了两个重要发现。首先,我们的噻唑系列中的甲基占据了一个小的疏水亚口袋,与TAK-875没有相互作用。此外,激动活性显示与噻唑核心和末端苯环之间的二面角具有良好的相关性。这些结果促进了对配体结合口袋的了解,并可能有助于设计更有希望的FFA1激动剂。
-
Acetamides and benzamides that are useful in treating sexual dysfunction申请人:——公开号:US20040029887A1公开(公告)日:2004-02-12The present invention relates to the use of compounds of formula (I) 1 for the treatment of sexual dysfunction and to compositions containing compounds of formula (I) for the treatment of sexual dysfunction.本发明涉及使用式(I)的化合物治疗性功能障碍,以及含有式(I)化合物的组合物用于治疗性功能障碍。
-
Selectfluor-mediated oxidative methylenation of amide with <i>N</i>,<i>N</i>-dimethylpropanamide for <i>N</i>,<i>N</i>′-methylenebisamide synthesis作者:Yue Cao、Dongheng Zhou、Yongmin MaDOI:10.1139/cjc-2018-0181日期:2019.1
A simple and efficient approach for the synthesis of N,N′-methylenebisamides through a Selectfluor-mediated oxidative reaction of aromatic amides and N,N-dimethylpropanamide (DMP) is described. Remarkable results clearly reveal that DMP plays a dual role in this reaction, as both a one-carbon source and an environment-friendly solvent. Moreover, the process provides new strategies for the synthesis of bisamides with advantages of operationally simple, insensitive to atmospheric conditions, and good to high yields.
表征谱图
-
氢谱1HNMR
-
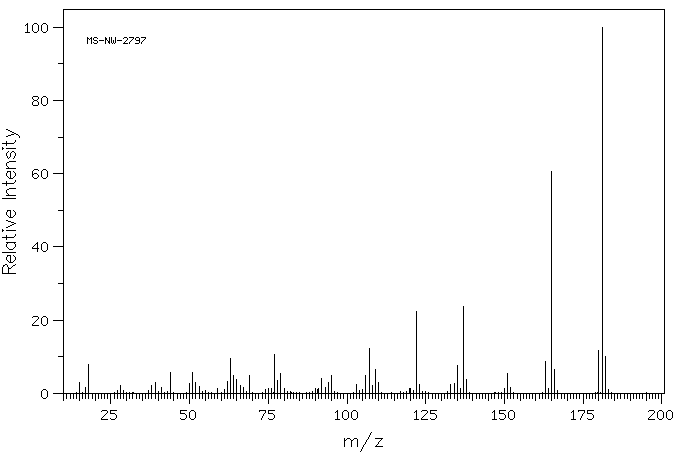
质谱MS
-
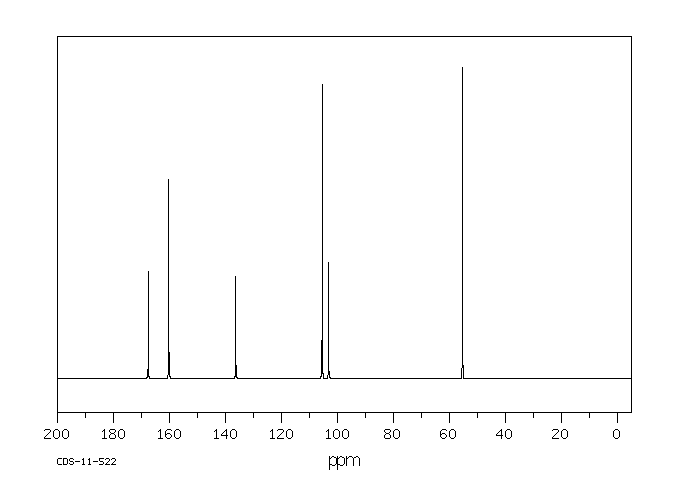
碳谱13CNMR
-
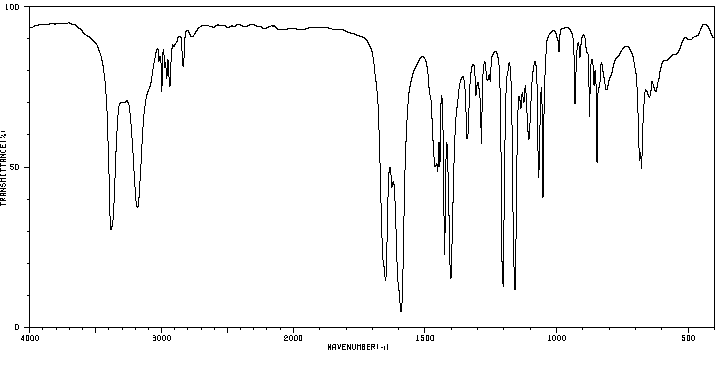
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息