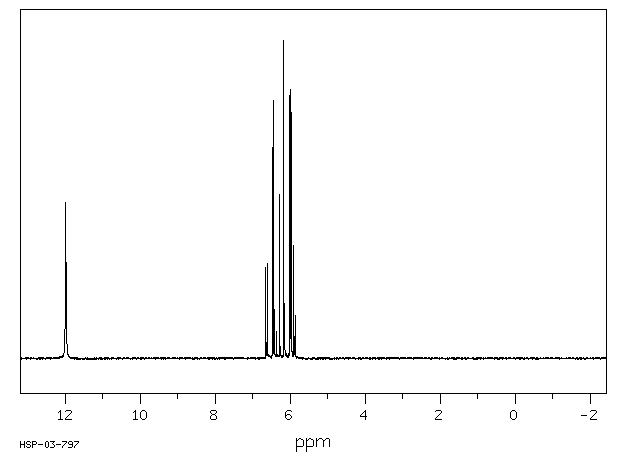
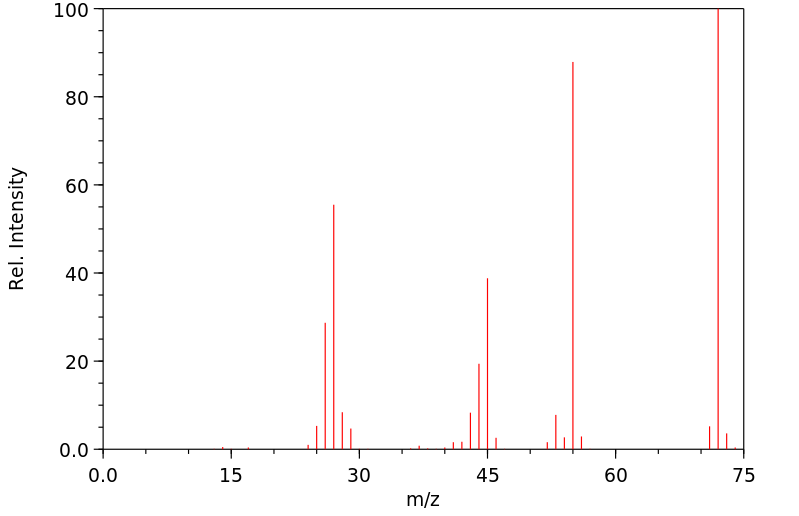
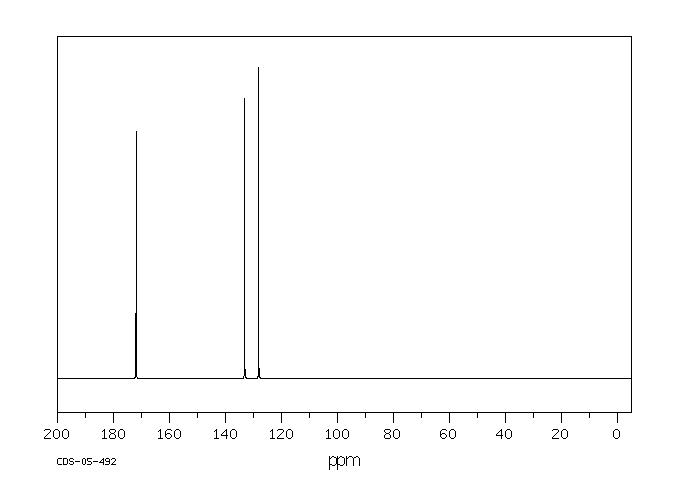
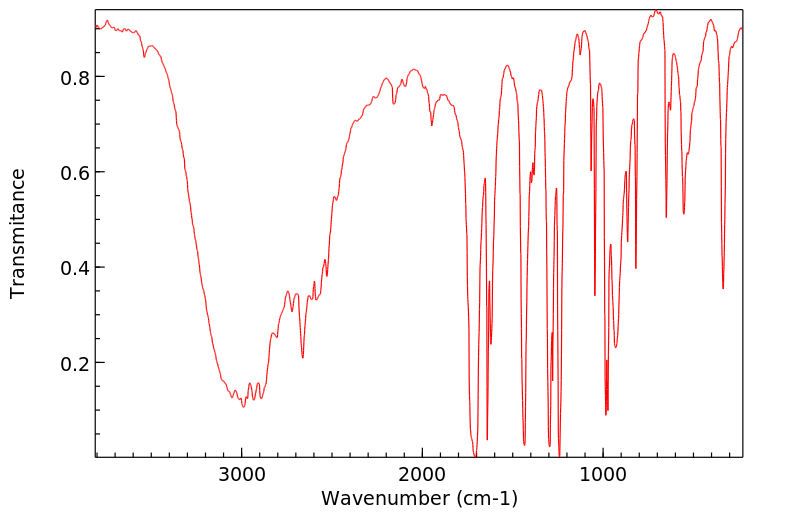
代谢
丙烯酸通过氧化途径迅速代谢成二氧化碳。丙烯酸的主要代谢途径似乎是一种次要的、非维生素B12依赖的丙酸代谢途径,包括与脂肪酸β-氧化类似的反应。在尿液中可以检测到比丙烯酸极性更高的、特性不明的物质。尿液中未检测到未代谢的丙烯酸,但发现了少量的3-羟基丙酸。没有检测到环氧化中间体。在体外(胃组织)和体内,丙烯酸与谷胱甘肽和非蛋白巯基的亲和力非常低。导致组织损伤的高剂量丙烯酸会引起少量巯基尿酸衍生物的形成。
Acrylic acid is rapidly metabolized by oxidative pathways to CO2. The main metabolic pathway of acrylic acid seems to be a secondary, non-vitamin-B12 dependent pathway of propionic acid metabolism consisting in reactions similar to fatty acid beta-oxidation. In urine poorly characterized substances of a higher polarity than those of acrylic acid are detected. Unmetabolized acrylic acid could not be detected in urine, however small amounts of 3-hydroxypropionic acid were found. Epoxide intermediates were not detected. In vitro (stomach tissue) and in vivo acrylic acid reacts with glutathione and non-protein sulfhydryls to a very low extent. High dosages of acrylic acid leading to tissue damage cause the formation of small amounts of mercapturic acid derivates.
来源:Hazardous Substances Data Bank (HSDB)