迷迭香酸 | 20283-92-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:171-175 °C (lit.)
-
比旋光度:+102~+110°(D/20℃)(c=0.2,C2H5OH)
-
沸点:694.7±55.0 °C(Predicted)
-
密度:1.33
-
溶解度:溶于乙醇、DMSO或二甲基甲酰胺至约25mg/mL。
-
LogP:0.871 (est)
-
物理描述:Solid
-
颜色/状态:Crystalline solid
-
蒸汽压力:1.1X10-13 mm Hg at 25 °C (est)
-
解离常数:pKa = 3.57 (est)
-
碰撞截面:195.6 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
-
稳定性/保质期:
按规定使用和贮存的不会分解,避免接触氧化物和空气。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:26
-
可旋转键数:7
-
环数:2.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:145
-
氢给体数:5
-
氢受体数:8
ADMET
安全信息
-
危险品标志:Xi,T
-
安全说明:S26,S37/39,S45
-
危险类别码:R25,R36/38
-
WGK Germany:3
-
海关编码:2932999099
-
RTECS号:GD8990000
-
储存条件:存储于干燥、阴凉、密闭处,并置于惰性气体中。
SDS
: 迷迭香酸
产品名称
1.2 鉴别的其他方法
(R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4- dihydroxyphenyl)lactic acid
3,4-Dihydroxycinnamic acid (R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl ester
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
当心 - 物质尚未完全测试。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: (R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4- dihydroxyphenyl)lactic acid
别名
3,4-Dihydroxycinnamic acid (R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl
ester
: C18H16O8
分子式
: 360.31 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
对空气敏感。 充气保存
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 深棕, 红色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 171 - 175 °C
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
对空气敏感。
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 静脉内的 - 老鼠 - 561 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
迷迭香酸是一种源自迷迭香植物(Rosmarinus officinalis L.)的重要天然成分,近年来因其广泛的健康益处而受到广泛关注。以下是关于迷迭香酸的一些重要信息:
1. 抗氧化作用- 清除自由基: 迷迭香酸通过与不饱和脂肪酸竞争性结合脂质过氧基来终止脂质过氧化的连锁反应,从而发挥强大的抗氧化效果。
- 结构特性: 邻二酚羟基是迷迭香酸清除自由基活性的基础。此外,C3位的共轭双键也增强了其抗氧化作用。
- 迷迭香酸对细菌细胞膜通透性、蛋白质代谢和DNA复制等具有一定的影响,从而发挥抑菌效果。
- 研究表明迷迭香酸可以增加小鼠脑海马回中标记的增值细胞数量,并可能通过海马回新生细胞增殖产生抗抑郁样作用。
- 食品行业: 可作为天然防腐剂,用于食品保存。
- 制药行业: 具有治疗心血管疾病、减肥降脂等功效的药物制备。
- 化妆品和个人护理产品: 如护发素、护肤油、香水等中添加迷迭香酸可以提供抗氧化保护和改善皮肤健康。
- 来源于唇形科迷迭香属植物迷迭香,主要成分包括具有抗氧化功能的酚类化合物。
- 强减肥降脂功效:研究表明其可以减少体内脂肪积累。
- 治疗心血管疾病: 抗氧化作用有助于预防心血管疾病的进展。
- 抗癌作用: 迷迭香酸可通过多种机制发挥抗肿瘤效果。
总之,迷迭香酸作为一种天然产物,具有多方面的健康益处,在未来的研究和应用中可能发挥更大的潜力。不过,进行具体研究时需要遵循相关法规并确保安全有效。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— prop-2-enyl (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxypropanoate 179462-78-3 C21H20O8 400.385 —— prop-2-enyl (2R)-3-(1,3-benzodioxol-5-yl)-2-[(E)-3-[3,4-bis(phenylmethoxy)phenyl]prop-2-enoyl]oxypropanoate 179129-01-2 C36H32O8 592.645 迷迭香酸-4-氧-葡萄糖苷 4-O-β-D-glucopyranosyl rosmarinic acid 910028-78-3 C24H26O13 522.463 丹参素 danshensu 76822-21-4 C9H10O5 198.175 alpha,3,4-三羟基-苯丙酸; 丹参素乳酸 danshensu 23028-17-3 C9H10O5 198.175 —— 3-(3,4-dimethoxy-phenyl)-2-hydroxy-propionic acid methyl ester —— C12H16O5 240.256 —— Prop-2-enyl 3-(1,3-benzodioxol-5-yl)-2-hydroxypropanoate 179128-92-8 C13H14O5 250.251 —— (R)-3-Benzo[1,3]dioxol-5-yl-2-hydroxy-propionic acid allyl ester 179128-94-0 C13H14O5 250.251 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 迷迭香酸甲酯 rosmarinic acid methyl ester 99353-00-1 C19H18O8 374.347 —— 3′-O-methylrosmarinic acid —— C19H18O8 374.347 —— dodecyl rosmarinate 1210482-25-9 C30H40O8 528.643 —— eicosyl rosmarinate 1210482-30-6 C38H56O8 640.858 —— octadecyl rosmarinate 1210482-28-2 C36H52O8 612.804 —— hexadecyl rosmarinate 1210482-26-0 C34H48O8 584.75 —— (R)-alpha-[3,4-Bis[4-(trifluoromethyl)benzyloxy]-trans-cinnamoyloxy]-3,4-bis[4-(trifluoromethyl)benzyloxy]benzenepropanoic acid methyl ester 1115211-08-9 C51H38F12O8 1006.84 —— 3-(4,5-Dihydroxy-2-nitrophenyl)-2-(3-(3,4-dihydroxyphenyl)acryloyloxy)propanoic acid 1020400-22-9 C18H15NO10 405.318 —— 3-(4,5-Dihydroxy-2-nitrophenyl)-2-(3-(4,5-dihydroxy-2-nitrophenyl)acryloyloxy)propanoic acid 1020400-23-0 C18H14N2O12 450.315 —— (+)-(S)-methyl 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate 136749-45-6 C10H12O5 212.202 甲基(2R)-3-(3,4-二羟基苯基)-2-羟基丙酸酯 danshensu methyl ester 457893-80-0 C10H12O5 212.202 alpha,3,4-三羟基-苯丙酸; 丹参素乳酸 danshensu 23028-17-3 C9H10O5 198.175 丹参素 danshensu 76822-21-4 C9H10O5 198.175 —— S-(-)-3-(3,4-Dihydroxyphenyl)lactic acid 42085-50-7 C9H10O5 198.175 —— (R)-2-(3-(3,4-dihydroxyphenyl)acryloyloxy)-3-(6-ethoxy-4,7-dioxo-4,7-dihydrooxepin-3-yl)propanoic acid 1261066-10-7 C20H18O10 418.357 —— 3-(3,4-dimethoxy-phenyl)-2-hydroxy-propionic acid methyl ester —— C12H16O5 240.256 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Isolation and Characterization of a Novel Tannase from a Metagenomic Library摘要:A novel gene (designated as tan410) encoding tannase was isolated from a cotton field metagenomic library by functional screening. Sequence analysis revealed that tan410 encoded a protein of 521 amino acids. SDS PAGE and gel filtration chromatography analysis of purified tannase suggested that Tan410 was a monomeric enzyme with a molecular mass of SS kDa. The optimum temperature and pH of Tan410 were 30 degrees C and 6.4. The activity was enhanced by addition of Ca2+, Mg2+ and Cd2+. In addition, Tan410 was stable in the presence of 4 M NaCl. Chlorogenic acid, rosmarinic acid, ethyl ferulate, tannic acid, epicatechin gallate and epigallocathchin gallate were efficiently hydrolyzed by recombinant tannase. All of these excellent properties make Tan410 an interesting enzyme for biotechnological application.DOI:10.1021/jf104394m
-
作为产物:描述:参考文献:名称:Pabsch, K.; Petersen, M.; Rao, N. N., Recueil des Travaux Chimiques des Pays-Bas, 1991, vol. 110, # 5, p. 199 - 205摘要:DOI:
-
作为试剂:描述:参考文献:名称:The Role of Rosmarinic Acid on the Bioproduction of Gold Nanoparticles as Part of a Photothermal Approach for Breast Cancer Treatment摘要:乳腺癌是对社会造成高负担的恶性肿瘤,其影响推动了对新型诊断和治疗工具的不断探索。在最近的治疗方法中,光热疗法(PTT)是一种高潜力的策略,通过光源照射后引起肿瘤细胞死亡。此外,将近红外(NIR)辐射与金纳米粒子(AuNPs)作为光热增强剂相结合,可以提高PTT的效果。本文报道了一种使用迷迭香酸(RA)合成AuNPs的替代合成方法。 RA浓度有所变化,并评估其对AuNPs物理化学和光学特性的影响。结果表明,RA浓度对AuNPs特性起着积极作用,允许优化平均大小和最大吸收峰。此外,本文探讨的合成方法使我们能够获得负电荷的AuNPs,其大小有利于在肿瘤部位局部粒子积累,并在NIR区域内具有最大吸收峰。此外,AuNPs在体外和体内均安全。总之,合成的AuNPs具有有利的特性,可作为将AuNPs与NIR激光相结合用于乳腺癌治疗的PTT系统的一部分。DOI:10.3390/biom12010071
文献信息
-
Kinetic Study on the Free Radical-Scavenging and Vitamin E-Regenerating Actions of Caffeic Acid and Its Related Compounds作者:Keishi Ohara、Yoko Ichimura、Kumi Tsukamoto、Mayumi Ogata、Shin-ichi Nagaoka、Kazuo MukaiDOI:10.1246/bcsj.79.1501日期:2006.10A kinetic study involving 4-hydroxycinnamic acid derivatives (HCAs) was performed in order to clarify the mechanism for free radical-scavenging and vitamin E-regenerating. The second-order rate con...
-
[EN] SYNTHESIS OF OLIGOMERIC NEOLIGNANS AND THEIR USE<br/>[FR] SYNTHÈSE DE NÉOLIGNANES OLIGOMÈRES ET LEURS APPLICATIONS申请人:UNIV COLUMBIA公开号:WO2010074764A1公开(公告)日:2010-07-01Compounds having the structure formule (I) and their uses are described herein.具有结构式(I)的化合物及其用途在此描述。
-
MYOCARDIAL REGENERATION PROMOTING COMPOUNDS, PREPARATION METHOD THEREOF, PHARMACEUTICAL COMPOSITION, AND THEIR USE申请人:GENHEALTH PHARMA CO., LTD.公开号:US20200317602A1公开(公告)日:2020-10-08The present invention discloses a novel 3-aryl-2-propen-1-one series derivative and the synthesis processes thereof. Besides, the present invention also discloses the series derivative as a pharmaceutical composition and their use for promoting myocardial regeneration.
-
Myricetin, rosmarinic and carnosic acids as superior natural antioxidant alternatives to α-tocopherol for the preservation of omega-3 oils作者:Romain Guitard、Jean-François Paul、Véronique Nardello-Rataj、Jean-Marie AubryDOI:10.1016/j.foodchem.2016.06.038日期:2016.12transfer, number of radicals scavenged per antioxidant molecule, BDE and formation of antioxidant dimers from the primary radicals play an important role regarding the antioxidant activity of phenols. Based on this, it is finally shown that myricetin, rosmarinic and carnosic acids are more efficient than α-tocopherol and synthetic antioxidants for the preservation of omega-3 oils.
-
Derivatization of Rosmarinic Acid Enhances its in vitro Antitumor, Antimicrobial and Antiprotozoal Properties作者:Silvia Bittner Fialová、Martin Kello、Matúš Čoma、Lívia Slobodníková、Eva Drobná、Ivana Holková、Mária Garajová、Martin Mrva、Vlastimil Zachar、Miloš LukáčDOI:10.3390/molecules24061078日期:——
On its own, rosmarinic acid possesses multiple biological activities such as anti-inflammatory, antimicrobial, cardioprotective and antitumor properties, and these are the consequence of its ROS scavenging and inhibitory effect on inflammation. In this study, two quaternary phosphonium salts of rosmarinic acid were prepared for the purpose of increasing its penetration into biological systems with the aim of improving its antimicrobial, antifungal, antiprotozoal and antitumor activity. The synthetized molecules, the triphenylphosphonium and tricyclohexylphosphonium salts of rosmarinic acid, exhibited significantly stronger inhibitory effects on the growth of HCT116 cells with IC50 values of 7.28 or 8.13 μM in comparison to the initial substance, rosmarinic acid (>300 μM). For the synthesized derivatives, we detected a greater than three-fold increase of activity against Acanthamoeba quina, and a greater than eight-fold increase of activity against A. lugdunensis in comparison to rosmarinic acid. Furthermore, we recorded significantly higher antimicrobial activity of the synthetized derivatives when compared to rosmarinic acid itself. Both synthetized quaternary phosphonium salts of rosmarinic acid appear to be promising antitumor and antimicrobial agents, as well as impressive molecules for further research.
独立的迷迭香酸具有多种生物活性,如抗炎、抗菌、心脏保护和抗肿瘤特性,这些是其清除ROS和抑制炎症作用的结果。在这项研究中,为了增加其在生物系统中的渗透性以提高其抗菌、抗真菌、抗原虫和抗肿瘤活性,制备了两种迷迭香酸的季铵磷盐。合成的分子,三苯基磷和三环己基磷盐的迷迭香酸,在抑制HCT116细胞生长方面表现出显著更强的效果,IC50值为7.28或8.13μM,而原始物质迷迭香酸(>300μM)。对于合成的衍生物,我们检测到对棘阿米巴和卢格登氏棘阿米巴的活性增加了三倍以上,对比迷迭香酸,对A. lugdunensis的活性增加了八倍以上。此外,与迷迭香酸相比,我们记录到合成的衍生物具有显著更高的抗菌活性。迷迭香酸的两种合成季铵磷盐似乎是有前途的抗肿瘤和抗菌剂,也是进一步研究的令人印象深刻的分子。
表征谱图
-
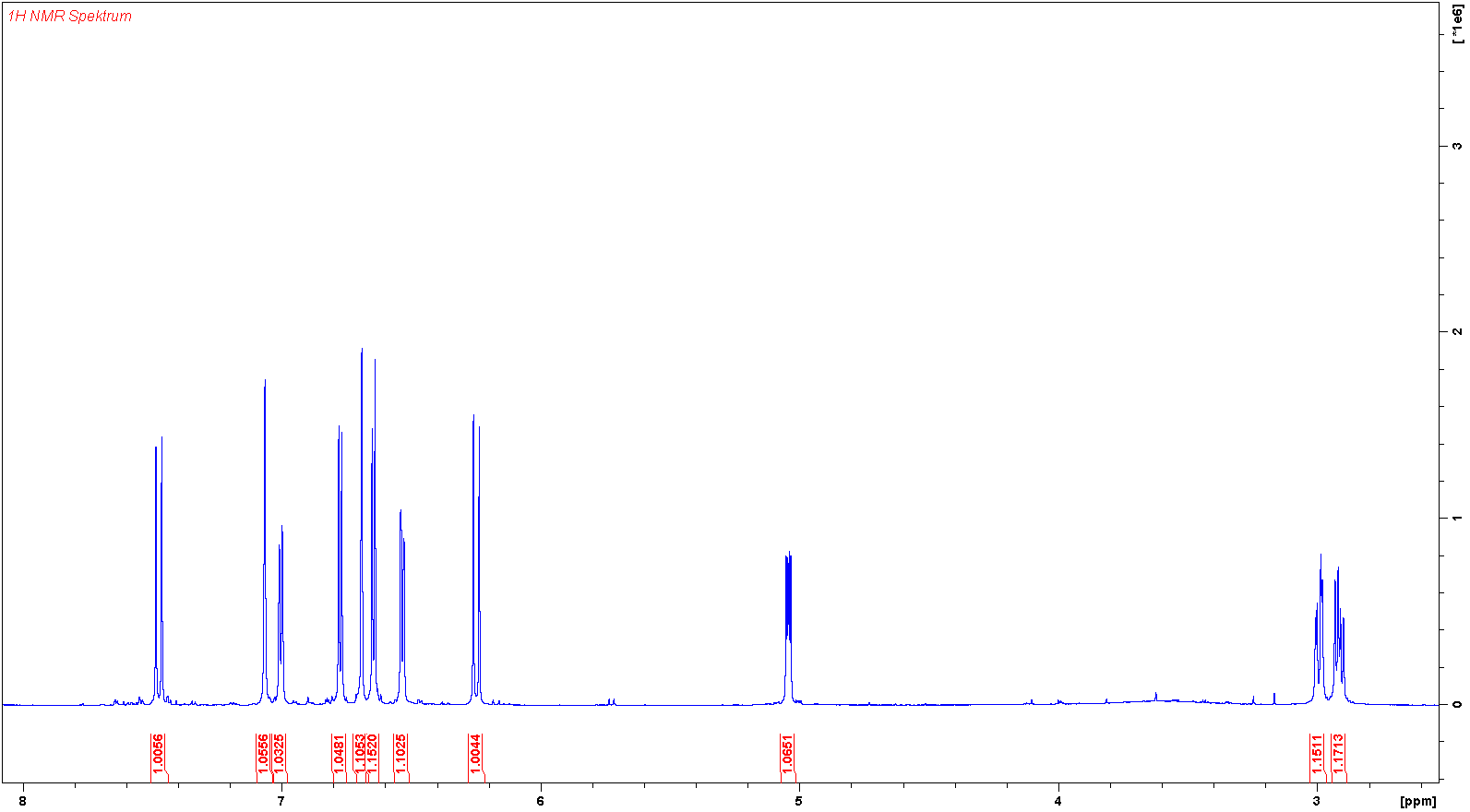
氢谱1HNMR
-
质谱MS
-
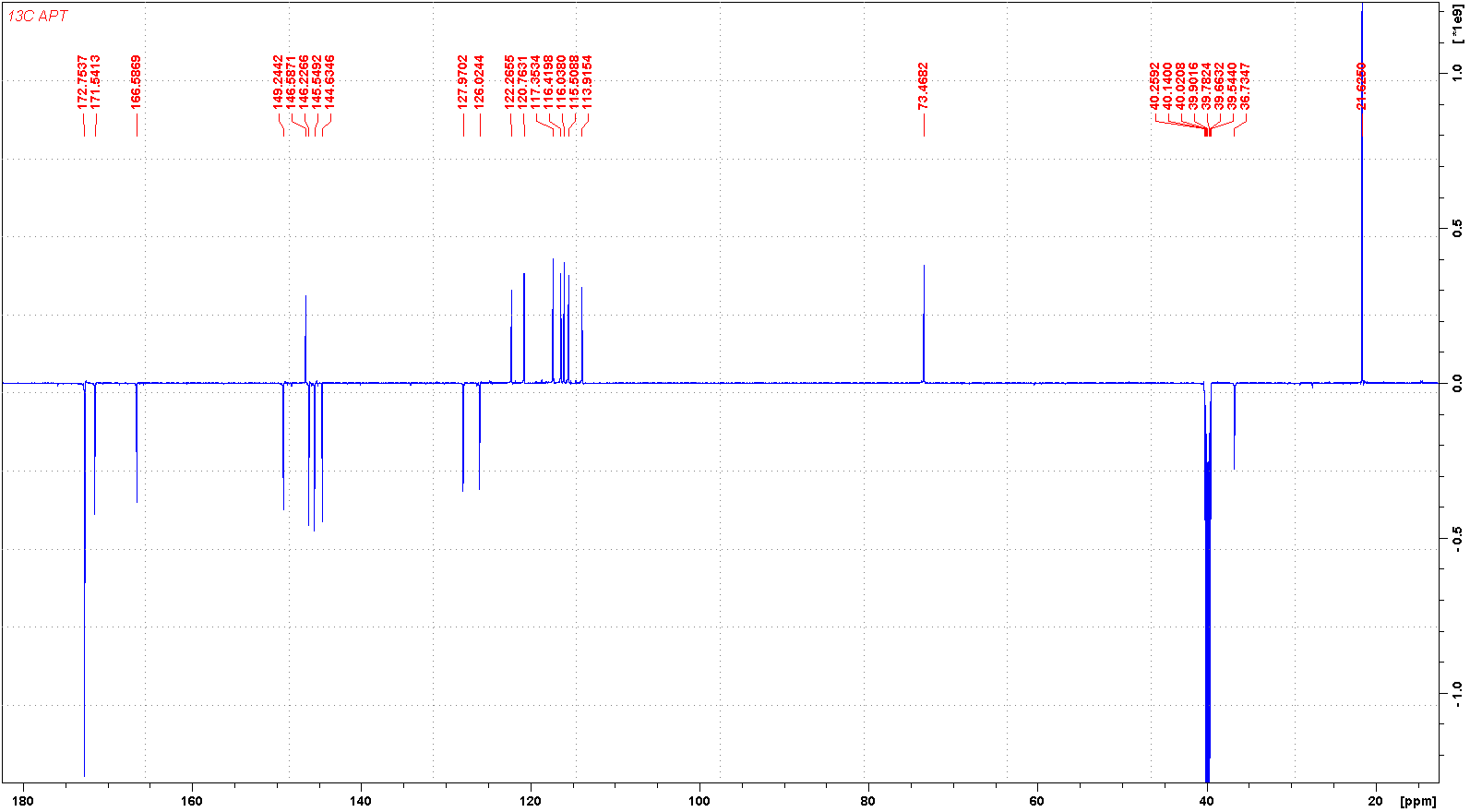
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息