天然橡胶 | 78-79-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:323-329 °C(lit.)
-
沸点:34 °C(lit.)
-
密度:0.681 g/mL at 25 °C(lit.)
-
蒸气密度:2.35 (vs air)
-
闪点:−65 °F
-
溶解度:0.7克/升
-
最大波长(λmax):231nm(neat)(lit.)
-
介电常数:2.1(25℃)
-
LogP:2.42 at 20℃
-
物理描述:Isoprene, stabilized appears as a clear colorless liquid with a petroleum-like odor. Density 5.7 lb / gal. Flash point -65°F. Boiling point 93°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a closed container, the container may rupture violently. Less dense than water and insoluble in water. Vapors heavier than air.
-
颜色/状态:Colorless volatile liquid
-
气味:Petroleum-lke
-
味道:Tasteless
-
蒸汽密度:2.35 (NTP, 1992) (Relative to Air)
-
蒸汽压力:550 mm Hg at 25 °C
-
大气OH速率常数:1.01e-10 cm3/molecule*sec
-
稳定性/保质期:
Stable under recommended storage conditions. Contains the following stabilizer(s): 4-tert-Butylpyrocatechol (>/= 100 to </= 150 ppm)
-
自燃温度:743 °F (395 °C)
-
分解:Decomposes at 120 °C.
-
粘度:0.3 mm²/s at 20-25 °C
-
燃烧热:-18,848 Btu/lb = -10,471 cal/g = -438.4X10+5 J/kg
-
汽化热:26.39 kJ/mol at 25 °C; 25.87 kJ/mol at 34 °C
-
表面张力:16.9 dynes/cm = 0.0169 N/m at 20 °C (liquid)
-
聚合:The substance polymerizes due to heating and under the influence of many materials. This generates fire or explosion hazard.
-
折光率:Index of refraction: 1.42160 at 20 °C/D
-
保留指数:502.2;504;506;504;496;506;508;507;507;504;505;504;508.6;506;502.95;500;503;502;503;504;502;503;503;503;504
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F+,T
-
安全说明:S45,S53,S61
-
危险类别码:R68,R45,R52/53,R12
-
WGK Germany:1
-
海关编码:4002601000
-
危险品运输编号:UN 1218 3/PG 1
-
危险类别:3
-
RTECS号:NT4037000
-
包装等级:I
-
危险标志:GHS02,GHS08,GHS09
-
危险性描述:H224,H341,H350,H411
-
危险性防范说明:P201,P210,P280,P308 + P313,P370 + P378,P403 + P235
SDS
制备方法与用途
异戊二烯常温下是一种无色易挥发、刺激性油状液体,不溶于水,溶于苯,易溶于乙醇、乙醚和丙酮。它与空气形成爆炸性混合物,爆炸极限大于1.6%。由于含有共轭双键,异戊二烯的化学性质活泼,容易自身聚合或与其他不饱和化合物共聚合,能与许多物质发生反应生成新的化合物。
高纯异戊二烯用途高纯异戊二烯主要用于生产异戊二烯橡胶、丁基橡胶、SIS(苯乙烯-异戊二烯-苯乙烯共聚物)和SEPS(SIS的加氢产品),也可用于制造专用化学品,如维生素、医药、香料、环氧固化剂。
化学性质无色易挥发、刺激性油状液体。不溶于水,溶于苯,并且易溶于乙醇、乙醚、丙酮。
用途 主要用途异戊二烯是合成橡胶的重要单体,其用量占异戊二烯总产量的95%。主要用于合成异戊橡胶,产量仅次于丁苯橡胶和顺丁橡胶而居合成橡胶的第三位。此外,还用于合成丁基橡胶的一种共聚单体,以改进丁基橡胶的硫化性能,但用量很少。异戊二烯还用于合成树脂、液体聚异戊二烯橡胶等,并用于制造农药、医药、香料及黏结剂。
合成用途主要用于生产性能接近天然橡胶的聚异戊二烯橡胶,也是丁基橡胶和SIS热塑性弹性体的第二单体。合成橡胶的重要单体,用量占异戊二烯总产量的95%,主要用于合成异戊橡胶。还用作合成丁基橡胶的一种共聚单体,以改进丁基橡胶的硫化性能。用于合成树脂、液体聚异戊二烯橡胶等。近年来,还用于合成里那醇、角鲨烯等,这些是进一步合成香料、药品、农药等的中间体。
生产方法 来源异戊二烯是碳五馏分的重要组分。主要来自炼厂催化裂化汽油得到的异戊烷脱氢和异戊烯脱氢,以及各种石油原料经裂解制乙烯时的副产物。碳五馏分沸点为27.9-49.3℃。
提纯方法碳五馏分组成复杂,直接精馏难以得到纯度较高的异戊二烯产品,通常采取萃取精馏及共沸精馏法由裂解碳五馏分中分离高纯度异戊二烯。已经工业化的萃取精馏溶剂有乙腈、二甲基甲酰胺和N-甲基吡咯烷酮。除碳五馏分分离得到异戊二烯外,工业上还采用合成法生产。
安全分类 分类易燃液体
毒性分级低毒
急性毒性吸入 - 大鼠 LC50: 180克/立方米/4小时;吸入 - 小鼠 LC50: 139克/立方米/2小时
爆炸物危险特性与空气混合可爆
可燃性危险特性遇明火、高温、氧化剂易燃;燃烧产生刺激烟雾
储运特性库房通风低温干燥;与氧化剂、酸类分开存放
灭火剂干粉、干砂、二氧化碳、泡沫
职业标准TWA 100 毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异丁烯 isobutene 115-11-7 C4H8 56.1075 2-甲基-丁烯 2-Methyl-1-butene 563-46-2 C5H10 70.1344 1,4-戊二烯 1,4-Pentadiene 591-93-5 C5H8 68.1185 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 异丁烯 isobutene 115-11-7 C4H8 56.1075 2-甲基-丁烯 2-Methyl-1-butene 563-46-2 C5H10 70.1344 —— 1-chloro-2-methyl-1,3-butadiene 98451-15-1 C5H7Cl 102.564 —— 2-chloromethyl-1,3-butadiene 4075-28-9 C5H7Cl 102.564 —— 2-methylene-3-buten-1-ol 13429-21-5 C5H8O 84.1179 2-(溴甲基)丁-1,3-二烯 2-(bromomethyl)buta-1,3-diene 23691-13-6 C5H7Br 147.015
反应信息
-
作为反应物:描述:参考文献:名称:硫代苯酚在烯基亚环丙烷中的加成反应。卡拉哈那酮的新型合成摘要:发现将硫酚加到链烯基亚烷基环丙烷(例如3、14)具有很高的区域选择性和立体选择性,从而导致-(或-起始原料中1,4-双键的添加导致乙烯基硫化物加成物(例如4、15)。。向(1)中加入硫酚导致硫化乙烯(16)和(17)的混合物经历Cope重排,产生环庚二烯(18);水解(18),然后使卡拉哈那酮(19)成为日本啤酒花的有臭成分。和柏树油。DOI:10.1016/s0040-4039(00)87118-x
-
作为产物:描述:参考文献:名称:DE267040摘要:公开号:
-
作为试剂:描述:茴香烯 、 methyl 6-cyano-9-phenanthrenecarboxylate 在 天然橡胶 作用下, 以 苯 为溶剂, 反应 2.5h, 生成 (Z)-茴香脑 、 methyl (1S,2R,10bS)-8-cyano-1-(4-methoxyphenyl)-2-methyl-2,2a-dihydro-1H-cyclobuta[l]phenanthrene-10b-carboxylate 、参考文献:名称:(E)-3-(4-Methoxyphenyl)-3-pentenyl 6-Cyano-9-phenanthrenecarboxylate 的分子内光环加成。温度和溶剂影响摘要:(E)-3-(4-Methoxyphenyl)-3-pentenyl 6-cyano-9-phenanthrenecarboxylate 以及相应的 9-phenanthrenecarboxylate 表现出激发态发射,并产生 [2+2] 与菲 9 双键的光环加合物, 10键(环丁烷)和酯羰基(氧杂环丁烷)。基于荧光和环加成的量子效率的温度和溶剂依赖性以及激基复合物中间体的猝灭剂的影响,提出了多个激基复合物中间体对这些现象的参与。DOI:10.1246/bcsj.67.1434
文献信息
-
Boron Lewis Acid-Catalyzed Regioselective Hydrothiolation of Conjugated Dienes with Thiols作者:Gautam Kumar、Zheng-Wang Qu、Soumen Ghosh、Stefan Grimme、Indranil ChatterjeeDOI:10.1021/acscatal.9b04647日期:2019.12.6tris(pentafluorophenyl)borane, B(C6F5)3, and BF3·Et2O are shown to catalyze the regioselective hydrothiolation of a wide range of terminal 1-aryl-1,3-dienes. In the case of internal 1,3-dienes, B(C6F5)3 is by far the better catalyst than BF3·Et2O. The process features mild reaction conditions, broad scope, and low catalyst loading, and it can be scaled up quickly over a short reaction time. The reactions are rate-limited
-
Convergent Synthesis of Menaquinone-7 (MK-7)作者:Aneta Baj、Piotr Wałejko、Andrzej Kutner、Łukasz Kaczmarek、Jacek W. Morzycki、Stanisław WitkowskiDOI:10.1021/acs.oprd.6b00037日期:2016.6.17A practical synthesis of menaquinone-7 (MK-7, vitamin K2) in the all-trans form was designed. Stereoselective synthesis of MK-7 was achieved through a “1 + 6” convergent strategy by condensation of two building blocks, menadione monoprenyl derivative (fragment “1”) with hexaprenyl bromide (fragment “6”, 82%). Pd-catalyzed desulfonation with LiEt3BH (78%) was followed by oxidation of the hydroquinone设计了一种实用的全反式甲萘醌7(MK-7,维生素K 2)合成方法。MK-7的立体选择性合成是通过“ 1 + 6”会聚策略实现的,该策略通过将两个组成部分,甲萘醌单异戊二烯基衍生物(片段“ 1”)与六异戊烯基溴化物(片段“ 6”,82%)缩合而实现。用LiEt 3 BH(78%)进行Pd催化的脱硫,然后使用硝酸铈(IV)铵(72%)氧化对苯二酚部分。我们方法学中的主要挑战是通过偶联两个由反式,反式衍生的三烯基单元来制备全反式六异戊烯基溴化物-法尼醇。通过我们的方法完成了中试规模的制造。这种可扩展的方法是专门为从易于获得的中间体进行大规模公斤级生产而设计的。此外,所提出的方法避免了许多色谱纯化,并且允许相对成本有效的制造。此外,我们的合成产生了高纯度(99.9%)的最终产品MK-7,可将其用作膳食补充剂以及活性药物成分。
-
DESIGN, SYNTHESIS AND FUNCTIONAL CHARACTERIZATION OF ROTTLERIN ANALOGS申请人:KANTHASAMY ANUMANTHA G.公开号:US20110112182A1公开(公告)日:2011-05-12A method of synthesizing rottlerin analogs is described. The synthesis methods described are the first known method of synthesizing rottlerin analogs from commercially-available materials to produce cost effective analogs. Rottlerin analog structures made by the synthesis methods and methods of use for treating a neurological or inflammatory response mediated by protein kinase C (PKC) are further described.描述了一种合成罗特林类似物的方法。所描述的合成方法是从商业可获得的材料中合成罗特林类似物的首个已知方法,以生产具有成本效益的类似物。通过这些合成方法制备的罗特林类似物结构以及用于治疗由蛋白激酶C(PKC)介导的神经或炎症反应的方法也进一步描述了。
-
TBAHF<sub>2</sub> and TBAH<sub>2</sub>F<sub>3</sub> as Activating Agents of Organosilanes作者:Atsunori Mori、Akinori Fujita、Kazutaka Ikegashira、Yasushi Nishihara、Tamejiro HiyamaDOI:10.1055/s-1997-3276日期:1997.6The fluoride reagents, TBAH2F3 and TBAHF2 (TBA: tetrabutylammonium), are found to catalyze the reactions of hexamethyldisilane with 1,3-dienes, aliphatic aldehydes, and aromatic aldehydes to give 1,4-disilyl-2-butenes, α-silyl alcohols and pinacols, respectively. Using an equimolar amount of TBAHF2 and disilanes, reduction of carbonyl compounds occurs in place of the silyl group addition.
-
Catalytic regio- and stereoselective intermolecular [5+2] cycloaddition <i>via</i> conjugative activation of oxidopyrylium作者:Ling Zhang、Qiu Shi、Tongxiang Cao、Shifa ZhuDOI:10.1039/d0cc04309e日期:——A catalytic stereodivergent intermolecular [5+2] cycloaddition of maltol-type oxidopyrylium through conjugative activation was reported, which featured high stereoselectivity, good compatibility and mild conditions, providing a convenient access route to various seven-membered heterocycles in moderate to excellent yields. In addition, a discrete mechanism was proposed to illustrate the stereoselectivity据报道,麦芽酚型氧化性霉菌通过共轭激活催化立体发散的分子间[5 + 2]环加成反应,具有高立体选择性,良好的相容性和温和的条件,为中等至优异的收率提供了方便的途径通往各种七元杂环。此外,提出了一种离散机制来说明立体选择性。
表征谱图
-
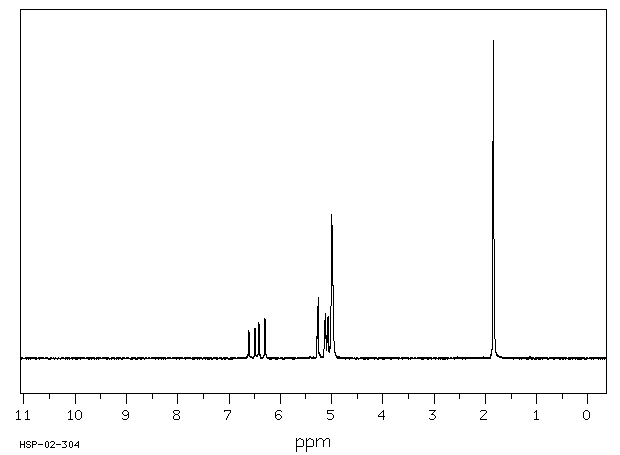
氢谱1HNMR
-
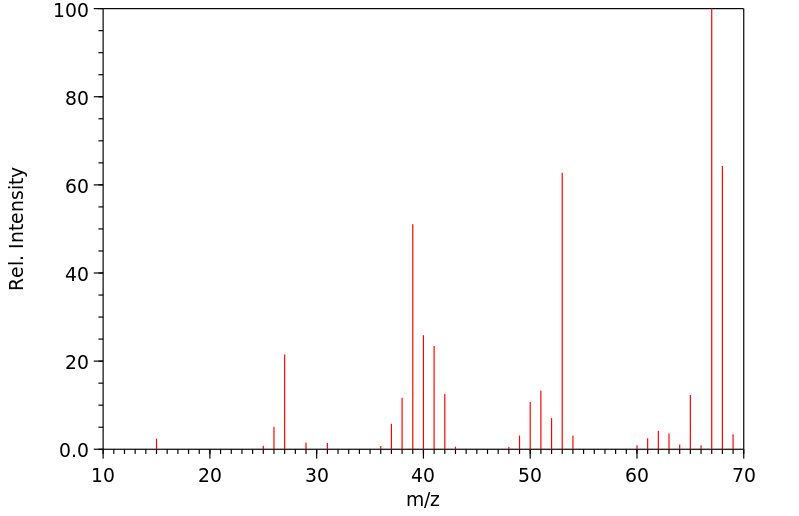
质谱MS
-
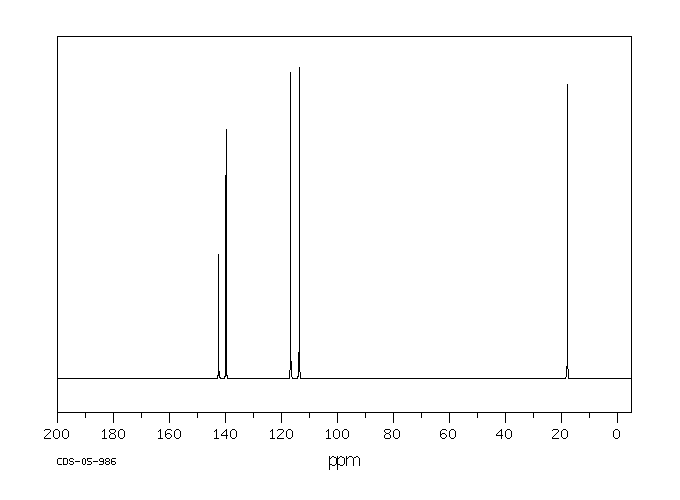
碳谱13CNMR
-
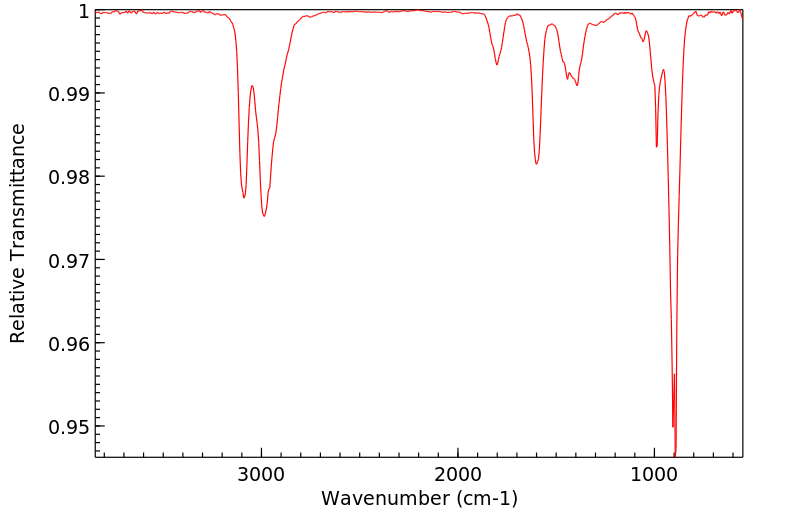
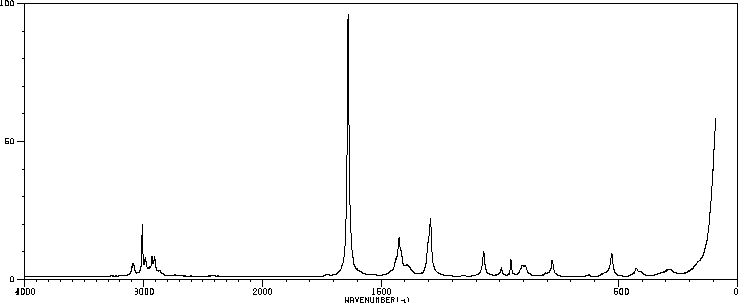
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息