1,1-二氯乙烯 | 75-35-4
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-122 °C (lit.)
-
沸点:30-32 °C (lit.)
-
密度:1.213 g/mL at 20 °C (lit.)
-
蒸气密度:3.46 (vs air)
-
闪点:−9 °F
-
溶解度:2.5g/l
-
介电常数:4.7(16℃)
-
暴露限值:TLV-TWA 5 ppm (~20 mg/m3) (ACGIH); TLV-STEL 20 ppm (ACGIH); carcinogenic ity: Animal Limited Evidence, Human Inad equate Evidence (IARC).
-
物理描述:Vinylidene chloride, stabilized appears as a clear colorless liquid with a chloroform-like odor. Flash point 0°F. Boiling point 99°F. Denser (at 10.1 lb / gal) than water and insoluble in water. Hence sinks in water. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Vapors heavier than air.
-
颜色/状态:Colorless liquid
-
气味:Mild, sweet odor resembling that of chloroform
-
蒸汽密度:3.25 (NTP, 1992) (Relative to Air)
-
蒸汽压力:600 mm Hg at 25 °C
-
亨利常数:0.03 atm-m3/mole
-
大气OH速率常数:1.09e-11 cm3/molecule*sec
-
稳定性/保质期:
-
易挥发且有毒,有自行聚合的倾向,并易被空气氧化。在氧化过程中会生成氯气、氯化氢、光气、甲醛及过氧化物,这些过氧化物又会促使单体进一步聚合。它还能与各种乙烯衍生物(如氯乙烯、苯乙烯等)共聚。因此需要加入阻聚剂或用氮、二氧化碳、碱的水溶液密封。铜或黄铜在某些条件下可能生成易爆炸的乙炔化合物。
-
在光或催化剂的作用下极易发生聚合反应,可与氯乙烯或丙烯腈等物质共聚。在空气中容易自氧化生成过氧化物,具有潜在的爆炸危险。这种过氧化物会缓慢分解成甲醛、光气和氯化氢。通常需要加入少量对苯二酚、酚类及烷基胺作稳定剂。40~50℃时与氯反应可生成1,1,2,2-四氯乙烷;在无水氯化铁或三氯化铝的存在下,会与氯化氢反应生成1,1,1-三氯乙烷。
-
皮肤和眼睛受到刺激。吸入高浓度蒸气会导致中枢神经系统麻痹、昏迷。长期低浓度吸入可能导致肝脏和肾脏损害,并可能对动物和人体产生致癌作用。因此使用时需注意通风。小鼠的致死浓度为25209.5 mg/m³,嗅觉阈浓度为1985 mg/m³。工作场所容许的最大蒸汽浓度应控制在40 mg/m³以下(以美国的标准为准)。
稳定
禁配物强氧化剂、酸类和碱类
避免接触的条件受热
聚合危害聚合
分解产物 -
-
自燃温度:1058 °F (570 °C)
-
分解:Hazardous decomposition products formed under fire conditions - Carbon oxides, hydrogen chloride gas.
-
粘度:0.3302 cP at 20 °C
-
腐蚀性:Vinylidene chloride may be corrosive or unstable in the presence of steel.
-
燃烧热:1095.9 kJ/mole at 25 °C
-
汽化热:26.48 kJ/mole at 25 °C
-
表面张力:24 dynes/cm at 15 °C (Inhibited)
-
电离电位:10.00 eV
-
聚合:When stored between -40 and +25 °C in the absence of inhibitor and in presence of air, vinylidene chloride rapidly absorbs oxygen with formation of a violently explosive peroxide. The latter initiates polymerization, producing an insoluble polymer which adsorbs the peroxide. Separation of this polymer in a dry state must be avoided, since if more than 15% of peroxide is present, the polymer may be detonable by slight shock or heat.
-
气味阈值:Odor threshold (air) = 2000-5500 mg/cu m
-
折光率:Index of refraction: 1.4249 at 20 °C/D
-
相对蒸发率:... The evaporation half-life of a dilute aqueous solution of vinylidene chloride (1 ppm w/w) in an open container stirred at 200 rpm at 25 °C /was/ 22 min, 90% of the compound was lost in 89 min.
-
保留指数:513;513;517;517;519;517.2;498;508.9;498;513;513.1;509.4;512;511;512;511;515;519;520;515
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
安全说明:S16,S29,S36/37,S45,S46,S7
-
危险品运输编号:UN 1303 3/PG 1
-
WGK Germany:3
-
危险类别:3
-
危险品标志:Xn,F+
-
危险类别码:R40,R12,R20
-
RTECS号:KV9275000
-
包装等级:I
-
危险标志:GHS02,GHS06,GHS08
-
危险性描述:H224,H301,H315,H319,H332,H351
-
危险性防范说明:P210,P281,P301 + P310,P305 + P351 + P338
-
储存条件:储存注意事项: - 储存在阴凉、通风良好的库房中。 - 远离火源和热源,库温不宜超过37℃。 - 包装需密封,避免与空气接触。 - 应与氧化剂、酸类及碱类分开存放,严禁混储。 - 不宜久存,以防变质。 - 使用防爆型照明和通风设施,并禁止使用易产生火花的机械设备和工具。 - 储区应配备泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 32040 |
| CAS: | 75-35-4 |
| 中文名称: | 1,1-二氯乙烯 |
| 英文名称: | 1,1-dichloroethylene;vinylidene chloride |
| 别 名: | 偏二氯乙烯、乙烯叉二氯 |
| 分子式: | C 2 H 2 Cl 2 ;CH 2 CCl 2 |
| 分子量: | 96.94 |
| 熔 点: | -122.6℃ |
| 密 度: | 相对密度(水=1)1.21; |
| 蒸汽压: | -28℃ |
| 溶解性: | 不溶于水 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体,带有不愉快气味 |
| 危险标记: | 7(中闪点易燃液体) |
| 用 途: | 用作辅聚剂、粘合剂和用于有机合成 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。 健康危害:主要影响中枢神经系统,并有眼及上呼吸道刺激症状。 急性中毒:短时间接触低浓度,眼及咽喉部烧灼感;浓度增高,有眩晕、恶心、呕吐甚至酩酊状;吸入高浓度还可致死。可致角膜损伤及皮肤灼伤。 慢性影响:长期接触,除粘膜刺激症状外,常伴有神经衰弱综合征。
二、毒理学资料及环境行为
毒性:对动物和人有致瘤作用。以吸入最为危险。 急性毒性:LD50200mg/kg(大鼠经口);LC5025210mg/m3,4小时(大鼠吸入);人吸入<5ppm,肝功能略有影响。 亚急性和慢性毒性:动物接触0.397g/m3和0.199g/m3,8小时/天,5天/周,数月后出现肝肾损害。接触低于0.099g/m3,出现轻度肝肾病变。 致突变性:微生物致突变:鼠伤寒沙门氏菌5pph。DNA损伤:大鼠吸入10ppm。 致癌性:IARC致癌性评论:动物阳性,人类无可靠数据。大鼠吸入55ppm×6小时/日×12月,肝血管肉瘤。 致畸性:大鼠吸入200ppm(妊娠)致畸胎作用。
危险特性:易燃,其蒸气与空气可形成爆炸性混合物。遇明火、高热能引起燃烧爆炸。受高热分解产生有毒的腐蚀性烟气。与氧化剂接触会猛烈反应。其蒸气比空气重,能在较低处扩散到相当远的地方,遇明火会引着回燃。 燃烧(分解)产物:一氧化碳、二氧化碳、氯化氢、光气。
3.现场应急监测方法:
4.实验室监测方法:
| 监测方法 | 来源 | 类别 |
| 吹扫捕集-气相色谱法 | 中国环境监测总站 | 水质 |
| 气相色谱法 | 《水和废水标准检验法》第19版译文,江苏省环境监测中心 | 水质 |
| 气相色谱法 | 《固体废弃物试验与分析评价手册》中国环境监测总站等译 | 固体废弃物 |
| 色谱/质谱法 | 美国EPA524.2方法 | 水质 |
5.环境标准:
| 前苏联 | 车间空气中有害物质的最高容许浓度 | 50mg/m 3 |
| 中国(GHZB1-1999) | 地面水环境质量标准(I、II、III类水域特定值) | 0.007mg/L |
| 日本(1993) | 环境标准 | 地面水:0.002mg/L 废水:0.02mg/L 土壤浸出液:0.02mg/L |
| 嗅觉阈浓度 | 500ppm |
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源。防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 废弃物处置方法:建议用焚烧法处理。废弃物和其它燃料混合焚烧,燃烧要充分,防止生成光气。焚烧炉排出的卤化氢通过酸洗涤器除去。
二、防护措施
呼吸系统防护:空气中浓度超标时,应该佩戴过滤式防毒面具(半面罩)。紧急事态抢救可撤离时,佩戴隔离式呼吸器。 眼睛防护:戴化学安全防护眼镜。 身体防护:穿防静电工作服。 手防护:戴橡胶手套。 其它:工作现场禁止吸烟、进食和饮水。工作毕,淋浴更衣。注意个人清洁卫生。
三、急救措施
皮肤接触:立即脱去被污染的衣着,用大量流动清水冲洗,至少15分钟。就医。 眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。就医。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 食入:误服者用水漱口,给饮牛奶或蛋清。就医。
灭火方法:尽可能将容器从火场移至空旷处。时节水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。用水灭火无效。
制备方法与用途
偏二氯乙烯是一种具有特殊气味的无色液体,易气化,沸点为31.7℃,折射率为1.4259,相对密度为1.2130,在水中几乎不溶,并能溶解于多种有机溶剂。
用途偏二氯乙烯即1,1-二氯乙烯,是制备二氯菊酸甲酯的中间体。该物质不仅自身可聚合,还能与氯乙烯、丙烯腈、丁二烯、甲基丙烯酸及甲基丙烯酸甲酯等多种化合物共聚,广泛应用于聚合物工业和电影胶片工业。
1,1-二氯乙烯为基础(含至少80%)的共聚物可以制得具有耐火性的偏氯纶。与丙烯腈、丁二烯、丙烯酸酯及苯乙烯等物质共聚后,可生成多种合成树脂,适用于制作纤维或薄膜,并可用于纸张或塑料薄膜的表面涂层。
聚偏二氯乙烯纤维能够用于制造织物、帐篷、防虫网和汽车坐垫。聚偏二氯乙烯薄膜透气性和透湿性较低,适合食品包装。此外,在与甲基丙烯酸及甲基丙烯酸甲酯共聚时,可用于电影胶片工业。
用途主要用来生产偏氯乙烯-氯乙烯、偏氯乙烯-丙烯腈和偏氯乙烯-丙烯酸酯类共聚物,并广泛用于合成纤维等制造领域。
生产方法工业上通常由氯乙烯与氯气反应生成1,1,2-三氯乙烷,再通过10%氢氧化钙水溶液脱除氯化氢而得偏二氯乙烯。原料消耗定额为:氯乙烯730千克/吨、氯870千克/吨、氢氧化钙750千克/吨和三氯乙烷1500千克/吨。
另一种方法是将氯乙烯与氯气在80~90℃条件下以铁屑为催化剂生成1,1,2-三氯乙烷,再将其与氢氧化钠在相同温度下反应脱去一分子氯化氢得到粗偏二氯乙烯,最后通过蒸馏即可获得成品。反应方程式如下:[ \text{CH}_2=\text{CHCl} + \text{Cl}_2 \rightarrow [\text{FeCl}_3] \text{CH}_2\text{Cl}-\text{CHCl}_2 + \text{NaOH} \rightarrow \text{CH}_2=\text{CCl}_2 ]
安全特性上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三氯乙烯 Trichloroethylene 79-01-6 C2HCl3 131.389 四氯乙烯 1,1,2,2-tetrachloroethylene 127-18-4 C2Cl4 165.834 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三氯乙烯 Trichloroethylene 79-01-6 C2HCl3 131.389 四氯乙烯 1,1,2,2-tetrachloroethylene 127-18-4 C2Cl4 165.834 氯乙烯 chloroethylene 75-01-4 C2H3Cl 62.4988 1,1-二氯-2-氟乙烯 1,1-dichloro-2-fluoro-ethene 359-02-4 C2HCl2F 114.934
反应信息
-
作为反应物:描述:参考文献:名称:Biesalski, Angewandte Chemie, 1924, vol. 37, p. 317摘要:DOI:
-
作为产物:参考文献:名称:Pathways of Chlorinated Ethylene and Chlorinated Acetylene Reaction with Zn(0)摘要:To successfully design treatment systems relying on reactions of chlorocarbons with zero-valent metals, information is needed concerning the kinetics and pathways through which transformations occur. In this study, pathways of chlorinated ethylene reaction with Zn(0) have been elucidated through batch experiments. Data for parent compound disappearance and product appearance were fit to pseudo-first-order rate expressions in order to develop a complete kinetic model. Results indicate that reductive beta-elimination plays an important role, accounting for 15% of tetrachloroethylene (PCE), 30% of trichloroethylene (TCE), 85% of cis-dichloroethylene (cis-DCE), and 95% of trans-dichloroethylene (trans-DCE) reaction. The fraction of PCE, TCE, trans-DCE, and cis-DCE transformation that occurs via reductive elimination increases as the two-electron reduction potential (E-2)for this reaction becomes more favorable relative to hydrogenolysis. In the case of PCE a nd TCE, reductive elimination gives rise to chlorinated acetylenes. Chloroacetylene and dichloroacetylene were synthesized and found to react rapidly with zinc, displaying products consistent with both hydrogenolysis and reduction of the triple bond. Surface area-normalized rate constants (k(SA)) for chlorinated ethylene disappearance correlate well with both one-electron (E-1) and two-electron (E-2) reduction potentials for the appropriate reactions. Correlation with E-2 allows prediction of the distribution of reaction products as well as the rate of disappearance of the parent compound.DOI:10.1021/es980252o
-
作为试剂:描述:N-溴代丁二酰亚胺(NBS) 、 3,3-二甲基-1-丁烯 在 新戊烷 、 二氯甲烷 、 1,1-二氯乙烯 作用下, 反应 1.0h, 生成 丁二酰亚胺 、 N-(2-bromo-3,3.-dimethyl-1-butyl)succinimide 、 3-溴丙酰异氰酸酯 、 alkaline earth salt of/the/ methylsulfuric acid参考文献:名称:Excited-state σ succinimidyl and glutarimidyl radicals: reversible ring opening摘要:DOI:10.1021/ja00389a058
文献信息
-
Synthesis of poly-functionalized pyrazoles under Vilsmeier-Haack reaction conditions作者:Aleksandr V. Popov、Valentina A. Kobelevskaya、Ludmila I. Larina、Igor B. RozentsveigDOI:10.24820/ark.5550190.p010.934日期:——Synthesis of 1,3-disubstituted 5-chloro-1H-pyrazole-4-carbaldehydes was achieved by formylation of the corresponding 5-chloro-1H-pyrazoles under Vilsmeier-Haack conditions.
-
PREPARATION METHOD AND USE OF COMPOUNDS HAVING HIGH INSECTICIDAL ACTIVITIES申请人:Li Zhong公开号:US20090111847A1公开(公告)日:2009-04-30The present invention discloses a kind of nitromethylene derivatives as well as their preparation method and their uses. The insecticidal activity tests show that the nitromethylene derivatives of the present invention not only show high insecticidal activities against insects with piercing-sucking type or scratching type mouthparts, such as aphid, leafhopper, plant hopper, thrips and white fly and their resistant strains, but also show high insecticidal activities against Lissorhoptrus oryzophilus , carmine spider mite, and they can also be used to prevent sanitary pest, and white ant.
-
COMPOUNDS AND USES THEREOF申请人:Yumanity Therapeutics, Inc.公开号:US20190330198A1公开(公告)日:2019-10-31The present invention features compounds useful in the treatment of neurological disorders. The compounds of the invention, alone or in combination with other pharmaceutically active agents, can be used for treating or preventing neurological disorders.本发明涉及用于治疗神经系统疾病的化合物。本发明的化合物可以单独或与其他药用活性剂结合使用,用于治疗或预防神经系统疾病。
-
Formation of 1,4,2-Dithiazolidines or 1,3-Thiazetidines from 1,1-Dichloro-2-nitroethene and Phenylthiourea Derivatives作者:Yian Feng、Minming Zou、Runjiang Song、Xusheng Shao、Zhong Li、Xuhong QianDOI:10.1021/acs.joc.6b01307日期:2016.11.41,4,2-dithiazolidine or 1,3-thiazetidine heterocycles was developed by reactions of phenylthioureas with 1,1-dichloro-2-nitroethene. The solvent has a significant influence on the type of product formation. 1,4,2-Dithiazolidines were formed in the aprotic solvent chloroform, while in the protic solvent ethanol, 1,3-thiazetidines were the main products.
-
Synthesis of 13-alkyl-gon-4-ones申请人:Smith; Herchel公开号:US03959322A1公开(公告)日:1976-05-25The preparation of 13-methylgon-4-enes and novel 13-polycarbonalkylgon-4-enes by a new total synthesis is described. 13-Alkylgon-4-enes having progestational, anabolic and androgenic activities are prepared by forming a tetracylic gonane structure unsaturated in the 1,3,5(10),9(11) and 14-positions, selectively reducing in the B- and C-rings, and converting the aromatic A-ring compounds so-produced to gon-4-enes by Birch reduction and hydrolysis.描述了通过新的全合成方法制备13-甲基孕-4-烯和新型13-聚碳烷基孕-4-烯。通过形成在1,3,5(10),9(11)和14-位置不饱和的四环孕烷结构,选择性地在B和C环中还原,并将所产生的芳香A环化合物转化为孕-4-烯,制备具有孕激素、合成激素和雄激素活性的13-烷基孕-4-烯。
表征谱图
-
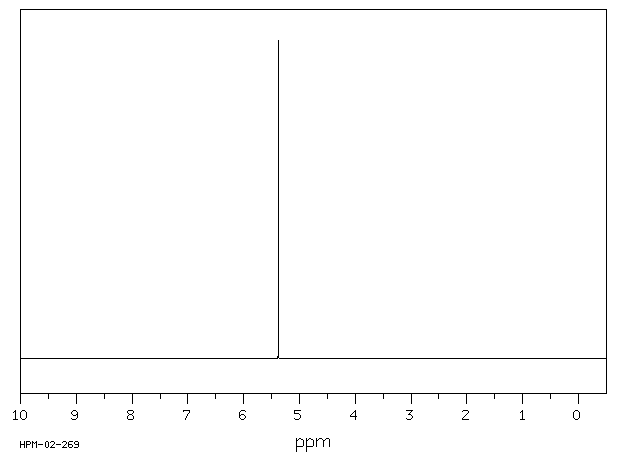
氢谱1HNMR
-
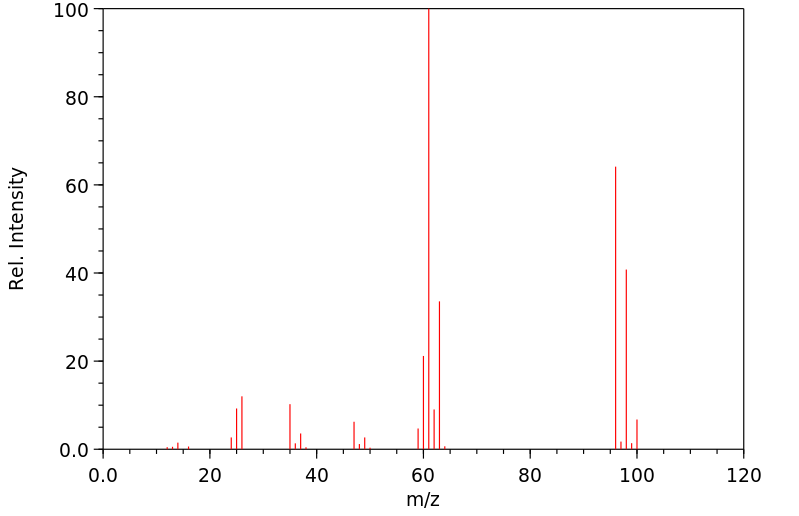
质谱MS
-
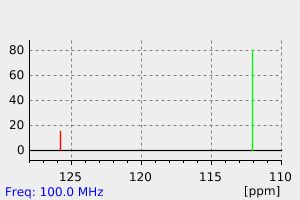
碳谱13CNMR
-
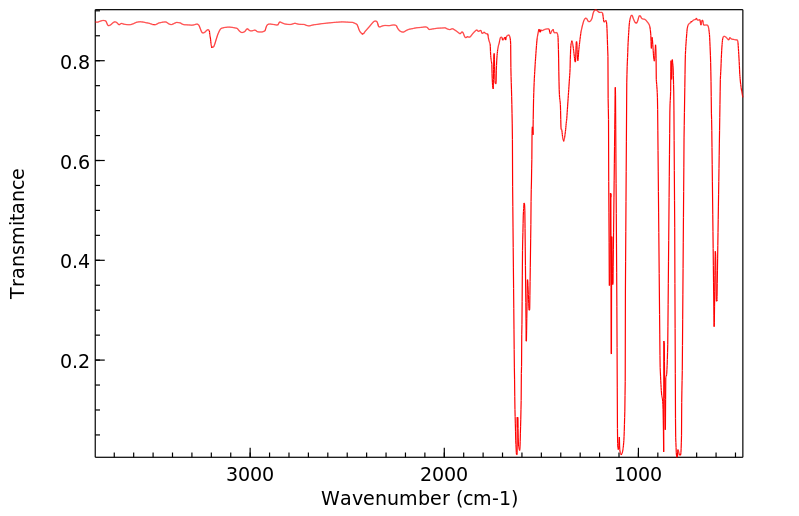
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息