三氯乙烯 | 79-01-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-86 °C
-
沸点:87 °C
-
密度:1.463 g/mL at 25 °C(lit.)
-
蒸气密度:4.5 (vs air)
-
闪点:90°C
-
溶解度:Soluble in acetone, ethanol, chloroform, ether (U.S. EPA, 1985), and other organic solvents including bromoform, carbon tetrachloride, methylene chloride, trichloroethylene, and tetrachloroethylene.
-
暴露限值:TLV-TWA 50 ppm (~270 mg/m3) (ACGIH), 100 ppm (MSHA and OSHA); TLV-STEL 200 ppm (ACGIH); ceiling 200 ppm (OSHA); carcinogenicity: Animal Lim ited Evidence, Human Inadequate Evidence (IARC).
-
介电常数:3.4(16℃)
-
LogP:2.53 at 20℃
-
物理描述:Trichloroethylene appears as a clear colorless volatile liquid having a chloroform-like odor. Denser than water and is slightly soluble in water. Noncombustible. Used as a solvent, fumigant, in the manufacture of other chemicals, and for many other uses.
-
颜色/状态:Colorless liquid (unless dyed blue)
-
气味:Ethereal odor
-
味道:Sweet burning taste
-
蒸汽密度:4.53 (NTP, 1992) (Relative to Air)
-
蒸汽压力:69 mm Hg at 25 °C
-
亨利常数:0.01 atm-m3/mole
-
大气OH速率常数:2.36e-12 cm3/molecule*sec
-
稳定性/保质期:
-
化学性质:不含稳定剂的三氯乙烯在空气中逐渐被氧化,生成光气、一氧化碳和氯化氢。也可能生成少量二聚物(六氯丁烯)。反应按游离基历程进行,光照和加热明显促进反应。有水分存在时,二氯乙酰氯分解成二氯代乙酸和氯化氢,分解生成的酸性物质会腐蚀金属。因此,工业用三氯乙烯通常需加入微量稳定剂如酚类(对苯二酚)、胺类或醇类等。添加稳定剂的三氯乙烯在空气、水分和光存在下,即使加热至130℃,也不与一般工业用金属材料作用。
-
三氯乙烯蒸气加热至700℃以上分解生成二氯乙烯、四氯乙烯、四氯化碳、氯仿以及氯甲烷的混合物。三氯乙烯蒸气与空气一起受强烈光照时完全氧化成二氧化碳、氯化氢、一氧化碳和光气等。在铜盐存在下,加压加热175℃时,三氯乙烯与碱金属或碱土金属的氢氧化物水溶液或悬浊液反应生成羟基乙酸盐。冷时与盐酸及硝酸不反应,但加热时与浓硝酸激烈反应完全分解,通过控制条件可得到三氯硝基甲烷和一氯二硝基甲烷。在30~50℃条件下,在三氯化铝催化作用下,三氯乙烯与氯化氢反应生成1,1,1,2-四氯乙烷。在苛性碱存在下易发生脱氯化氢反应生成二氯乙炔,而二氯乙炔在空气中自燃并爆炸分解。碳酸钠及液态氨通常条件下与三氯乙烯不反应,但金属铝尤其是粉末状的金属铝能促使不含稳定剂的三氯乙烯分解,生成氯化氢的同时引发强烈爆炸或炭化。反应首先生成三氯化铝,作为Friedel Crafts催化剂促进三氯乙烯缩合生成五氯丁二烯,并进一步缩合成树脂、焦油。在三氯化铝存在下,三氯乙烯与氯仿反应可生成1,1,1,2,3,3-六氯丙烷;与四氯化碳反应则生成1,1,1,2,3,3,3-七氯丙烷。在过氧化物如过氧化苯甲酰存在下,加压加热至150~200℃可得到三氯乙烯的二聚物和三聚物。在三氯化铁催化下易发生氯化反应生成五氯乙烷和六氯乙烷。
-
稳定性
-
禁配物:强氧化剂、强还原剂、强碱、铝、镁
-
避免接触的条件:光照、紫外线
-
聚合危害:聚合
-
分解产物:氯化氢
-
-
自燃温度:420 °C (788 °F)
-
分解:Hazardous decomposition products formed under fire conditions - Carbon oxides, hydrogen chloride gas.
-
粘度:0.545 mPa s at 25 °C
-
腐蚀性:Non-corrosive
-
燃烧热:-6.56 kJ/g
-
汽化热:34.54 kJ/mol at 25 °C; 31.40 kJ/mol at 87.21 °C
-
表面张力:0.0264 N/m at 20 °C
-
电离电位:9.45 eV
-
气味阈值:5.00X10-1 mg/l (liquid) (detection in water)
-
折光率:Index of refraction: 1.4773 at 20 °C/D
-
相对蒸发率:3.0 (n-butyl acetate = 1, ethanol = 1.4, acetone = 5.7)
-
保留指数:683;687;690;694;691;688;675;691;696;702;710;698;687.4;691;680;680;676.6;693;694;691;688.1;687.7;691;681;681;691;710;672;686;686;689;673;680;689;710;683;690
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 25.0 ppm, STEL: 2.0 ppm
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:1,000 ppm
-
危险品标志:T
-
安全说明:S36/37,S45,S53,S61
-
危险类别码:R67,R45,R36/38,R52/53
-
WGK Germany:3
-
海关编码:2903220000
-
危险品运输编号:UN 1710 6.1/PG 3
-
危险类别:6.1
-
RTECS号:KX4550000
-
包装等级:III
-
危险标志:GHS07,GHS08
-
危险性描述:H315,H319,H336,H341,H350,H412
-
危险性防范说明:P201,P261,P273,P281,P305 + P351 + P338,P308 + P313
-
储存条件:储存注意事项: - 储存在阴凉、通风良好的库房中。 - 远离火源和热源,库温不超过32℃,相对湿度不超过80%。 - 包装需密封,避免与空气接触。 - 应与氧化剂、还原剂、碱类、金属粉末及食用化学品分开存放,切忌混储。 - 不宜大量储存或久存。 - 配备相应种类和数量的消防器材。 - 储区应设有泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 61580 |
| CAS: | 79-01-6 |
| 中文名称: | 三氯乙烯 |
| 英文名称: | trichloroethylene |
| 别 名: | 乙炔化三氯 |
| 分子式: | C 2 HCl 3 ;Cl 2 CCHCl |
| 分子量: | 131.39 |
| 熔 点: | -87.1℃ 沸点:87.1℃ |
| 密 度: | 相对密度(水=1)1.46; |
| 蒸汽压: | 32℃ |
| 溶解性: | 不溶于水,溶于乙醇、乙醚,可混溶于多数有机溶剂。 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色透明液体,有似氯仿的气味 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作溶剂,用于脱脂、冷冻、农药、香料、橡胶工业、洗涤织物等 |
2、对环境的影响
该物质对环境有严重危害,应特别注意对空气、水环境及水源的污染。在对人类重要食物链中,特别是在水生生物体中发生生物蓄积。
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:本品主要对中枢神经系统有麻醉作用。亦可引起肝、肾、心脏、三叉神经损害。
二、毒理学资料及环境行为
毒性:有蓄积作用。
急性毒性:LD502402mg/kg(小鼠经口);LC5045292mg/m3,4小时(小鼠吸入);137752mg/m3,1小时(大鼠吸入);人吸入6.89g/m3×6分钟,粘膜刺激;人吸入5.38g/m3×120分钟,视力减退;人吸入400ppm嗅到有气味,轻微眼刺激;人吸入2000ppm,极强烈的气味,不能耐受。
亚急性和慢性毒性:大鼠吸入0.54g/m3,5小时/天,5天/周,3个月,神经传导速度减慢。
致突变性:DNA抑制:人淋巴细胞5mg/L。姊妹染色单体交换:人淋巴细胞178mg/L。
生殖毒性:大鼠吸入最低中毒浓度(TCL0):1800ppm(24小时)(孕1~20天),引起肌肉骨骼发育异常。小鼠吸入最低中毒浓度(TCL0):100ppm/7小时(5天,雄性),精子生成异常。
致癌性:IARC致癌性评论:动物阳性,人类不明确。
危险特性:遇明火、高热能引起燃烧爆炸。与强氧化剂接触可发生化学反应。受紫外光照射或在燃烧或加热时分解产生有毒的光气和腐蚀性的盐酸烟雾。
燃烧(分解)产物:一氧化碳、二氧化碳、氯化氢、光气。
3、现场应急监测方法
气体检测管法;便携式气相色谱法;水质检测管法
直接进水样气相色谱法(1,1,2-三氯乙烯)
气体速测管(北京劳保所产品、德国德尔格公司产品)
4、实验室监测方法
监测方法 来源 类别
顶空气相色谱法 GB/T17130-1997 水质
无泵型采样器气相色谱法 WS/T144-1999 作业场所空气
吡啶-碱比色法;
气相色谱法 《空气中有害物质的测定方法》(第二版),杭士平主编 空气
气相色谱法 《固体废弃物试验与分析评价手册》中国环境监测总站等译 固体废弃物
色谱/质谱法 美国EPA524.2方法 水质
5、环境标准
中国(TJ36-79) 车间空气中有害物质的最高容许浓度 30mg/m3
前苏联(1987) 环境空气中最高容许浓度 4.0mg/m3(一次值)
1.0mg/m3(日均值)
中国(待颁布) 饮用水源中有害物质的最高容许浓度 0.07mg/L
中国(GB8978-1996) 污水综合排放标准 一级:0.3mg/L
二级:0.6mg/L
三级:1.0mg/L
中国(GHZB1-1999) 地表水环境质量标准(I、II、III类水域) 0.005mg/L
日本(1993) 环境标准 地面水:0.03mg/L
废水:0.3mg/L
土壤浸出液:0.03mg/L
嗅觉阈浓度 250ppm
6、应急处理处置方法
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。大量泄漏:构筑围堤或挖坑收容。用泡沫覆盖,降低蒸气灾害。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
废弃物处置方法:建议用焚烧法处理。废弃物和其它燃料混合焚烧,燃烧要充分,防止生成光气。焚烧炉排出的卤化氢通过酸洗涤器除去。此外,从废料中回收三氯乙烯,再循环使用。
二、防护措施
呼吸系统防护:可能接触其蒸气时,应该佩戴自吸过滤式防毒面具(半面罩)。紧急事态抢救或撤离时,佩戴循环式氧气呼吸器。
眼睛防护:戴化学安全防护眼镜。
身体防护:穿防毒物渗透工作服。
手防护:戴防化学品手套。
其它:工作现场禁止吸烟、进食和饮水。工作毕,沐浴更衣。单独存放被毒物污染的衣服。洗后备用。注意个人清洁卫生。
三、急救措施
皮肤接触:立即脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。就医。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗,就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
食入:饮足量温水,催吐,就医。
灭火方法:消防人员须戴氧气呼吸器。喷水保持火场容器冷却,直至灭火结束。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。
制备方法与用途
根据提供的信息,以下是关于三氯乙烯的一些关键点:
生产方法-
乙炔法:
- 口服大鼠LD50为5650毫克/公斤;小鼠为2402毫克/公斤。
- 刺激数据表明对皮肤有重度刺激作用,对眼睛有中度刺激作用。
- 与空气混合可以爆炸。
- 受热或遇明火时燃烧并释放有毒氯化物气体。
- 时间加权平均容许浓度(TWA)为270毫克/立方米;短时间接触极限值(STEL)为405毫克/立方米。
- 储运要求:库房应保持通风、低温和干燥,并与其他化学品分开存放。
- 使用时必须配备适当的个人防护装备,如呼吸器和防护眼镜。
- 库存和运输中要避免高温环境,并采取防爆措施。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 四氯乙烯 1,1,2,2-tetrachloroethylene 127-18-4 C2Cl4 165.834 1,1-二氯乙烯 1,1-Dichloroethylene 75-35-4 C2H2Cl2 96.9439 反-1,2-二氯乙烯 trans-1,2-dichloroethylene 156-60-5 C2H2Cl2 96.9439 1,2-二氯乙烯 1,2-Dichloroethene 540-59-0 C2H2Cl2 96.9439 顺-1,2-二氯乙烯 cis-1,2-Dichloroethylene 156-59-2 C2H2Cl2 96.9439 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 四氯乙烯 1,1,2,2-tetrachloroethylene 127-18-4 C2Cl4 165.834 1,1-二氯乙烯 1,1-Dichloroethylene 75-35-4 C2H2Cl2 96.9439 顺-1,2-二氯乙烯 cis-1,2-Dichloroethylene 156-59-2 C2H2Cl2 96.9439 反-1,2-二氯乙烯 trans-1,2-dichloroethylene 156-60-5 C2H2Cl2 96.9439 1,2-二氯乙烯 1,2-Dichloroethene 540-59-0 C2H2Cl2 96.9439 2-溴-1,1-二氯乙烯 2-bromo-1,1-dichloroethylene 5870-61-1 C2HBrCl2 175.84
反应信息
-
作为反应物:参考文献:名称:Little, J., British Journal of Industrial Medicine, 1955, vol. 12, p. 304 - 308摘要:DOI:
-
作为产物:描述:参考文献:名称:Manufacture of halogenated olefins摘要:公开号:US02308489A1
-
作为试剂:参考文献:名称:Prelog; Stepan, Collection of Czechoslovak Chemical Communications, 1935, vol. 7, p. 93,101摘要:DOI:
文献信息
-
[EN] CATALYST AND PROCESS USING THE CATALYST FOR MANUFACTURING FLUORINATED HYDROCARBONS<br/>[FR] CATALYSEUR ET PROCÉDÉ UTILISANT LE CATALYSEUR POUR LA FABRICATION D'HYDROCARBURES FLUORÉS申请人:MEXICHEM FLUOR SA DE CV公开号:WO2018046928A1公开(公告)日:2018-03-15A catalyst comprising chromia and at least one additional metal or compound thereof and wherein the catalyst has a total pore volume of greater than 0.3 cm3/g and the mean pore diameter is greater than or equal to 90 Å, wherein the total pore volume is measured by N2 adsorption porosimetry and the mean pore diameter is measured by N2 BET adsorption porosimetry, and wherein the at least one additional metal is selected from Li, Na, K, Ca, Mg, Cs, Sc, Al, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Co, Rh, Ir, Ni, Pd, In, Pt, Cu, Ag, Au, Zn, La, Ce and mixtures thereof.
-
Halocarbon Encapsulation via Halogen···π Interactions in a Bispyrazole-Based Cryptand作者:Ashish Verma、Kapil Tomar、Parimal K. BharadwajDOI:10.1021/acs.cgd.8b01471日期:2019.1.2structures clearly revealed that halogen bonding (C–Cl/Br···π (pyrazole)) and hydrogen bonding (C–H···π(pyrazole)) interactions played a key role in stabilizing the halogenated guests inside the hydrophobic cavity of cryptand. At the same time, the cage is efficiently able to exclude hydrophilic solvent molecules, like, water and methanol, suggesting the hydrophobic nature of the cavity. Due to the comparably一种新的基于bispyrazole膨胀穴状配体经由具有160埃的内部腔体的席夫碱缩合反应合成3用疏水性性质。穴状分子具有富电子的多个吡唑环,可增强与客体分子的弱非共价相互作用。研究了穴状配体的主客体能力,用于包封最不活泼的卤素键供体分子(具有较小的σ孔大小),即CH 2 Cl 2,CHCl 3,CCl 4,C 2 HCl 3,C 2 H 4 Cl 2和C 2 H 4 Br 2。晶体结构分析清楚地表明,卤素键(C–Cl / Br··π(吡唑))和氢键(CH–··π(吡唑))的相互作用在稳定卤代物内部起着关键作用。穴状的疏水腔。同时,该笼子能够有效地排除亲水性溶剂分子,例如水和甲醇,表明该腔体具有疏水性。由于C 2 H 4 Br 2中的σ孔相对较大,因此它显示出与主体穴体最强的卤素键相互作用,而CH 2 Cl 2的相互作用最弱。σ孔尺寸最小的宾客。此外,穴状体能够根据客人的大小调节其中央腔。对于C
-
Flash Chemistry Using Trichlorovinyllithium: Switching the Reaction Pathways by High-resolution Reaction Time Control作者:Aiichiro Nagaki、Yusuke Takahashi、Andrea Henseler、Chika Matsuo、Jun-ichi YoshidaDOI:10.1246/cl.140980日期:2015.2.5High-resolution reaction time control in flow microreactors enables the reaction-pathway switching of trichlorovinyllithium generated by the H/Li exchange of trichloroethene. The method was successfully applied to the synthesis of 1,1,2-trichloroalkenes, 1-chloroalkynes, and unsymmetrically disubstituted ethynes.
-
Synthesis and Singlet Oxygen Reactivity of 1,2-Diaryloxyethenes and Selected Sulfur and Nitrogen Analogs作者:Gregory Nkepang、Praveen K. Pogula、Moses Bio、Youngjae YouDOI:10.1111/j.1751-1097.2012.01095.x日期:2012.5oxygen‐mediated drug release. Even though 1,2‐diaryloxyethenes look very simple, their synthesis was not an easy task. Previous methods are limited to symmetric molecules, lengthy step and low yield. We report on a facile synthetic method not only for 1,2‐diaryloxyethenes but also their sulfur and nitrogen analogs in yields ranging from 40 to 90% with more than 90% purity at the vinylation reaction
-
A novel entry to xanthones by an intramolecular Diels-Alder reaction involving 2-(1,2-dichlorovinyloxy) aryl dienones作者:Katerina Otrubova、Anne E. Fitzgerald、Neelakandha S. ManiDOI:10.1016/j.tet.2018.08.007日期:2018.9of synthetic methods are described in the literature for the preparation of xanthones—a prominent class of tricyclic molecules that occur widely in nature. Majority of these reported methods involve linking the two aromatic rings and forming the central pyrone ring using a variety of classical and non-classical cyclization strategies. In a conceptually different approach, we describe here a new xanthone
表征谱图
-
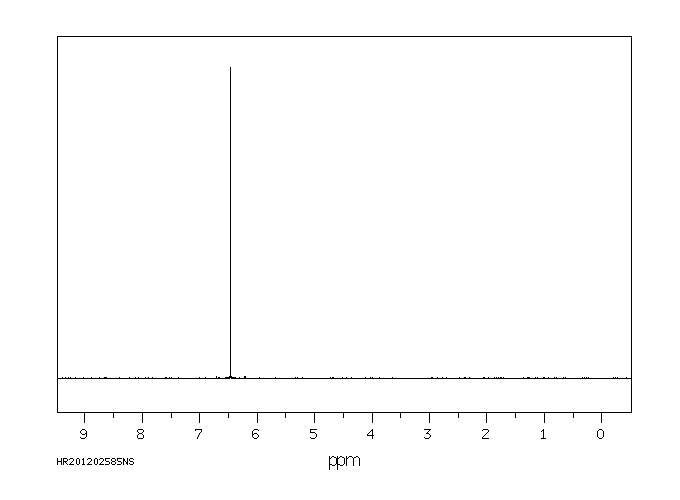
氢谱1HNMR
-
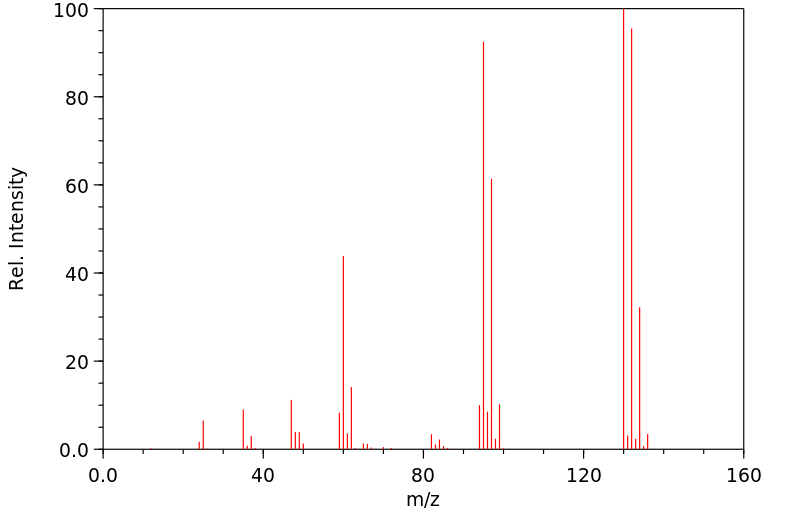
质谱MS
-
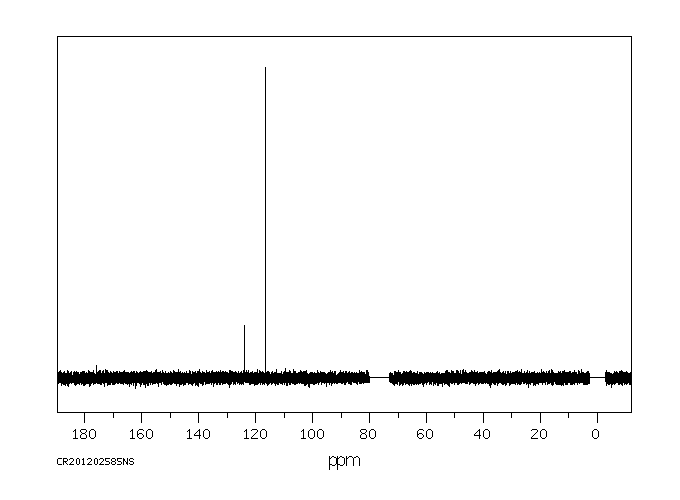
碳谱13CNMR
-
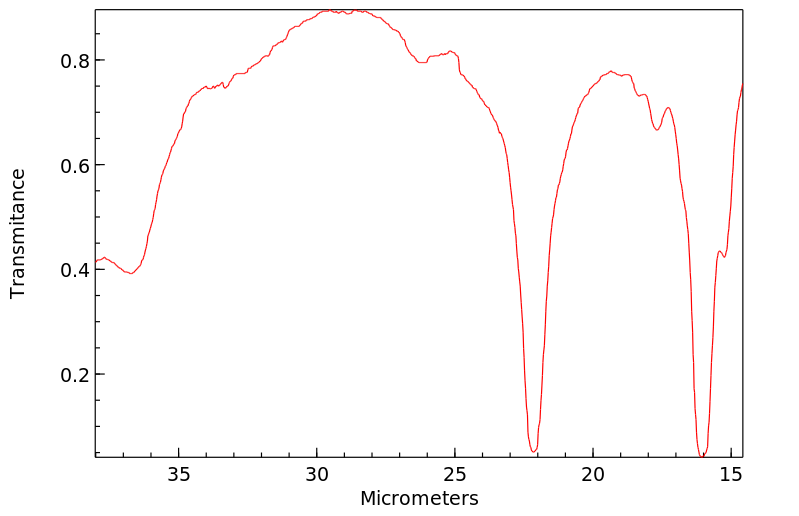
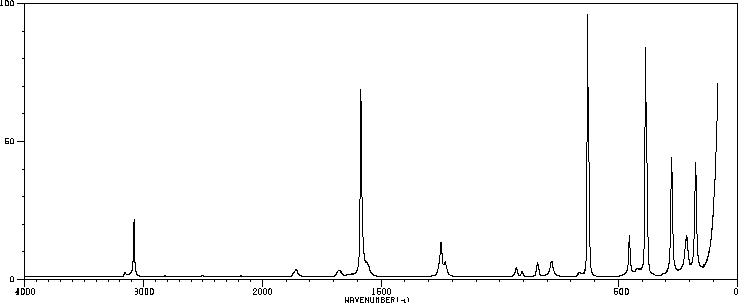
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息