1-苯基-2,4-戊二酮 | 3318-61-4
中文名称
1-苯基-2,4-戊二酮
中文别名
1-苯基-2,4-戊烷二酮
英文名称
1-phenyl-2,4-pentanedione
英文别名
1-phenylpentane-2,4-dione;1-Phenyl-2,4-pentandion;1-Phenyl-pentandion-(2,4);1-Phenyl-acetylaceton
CAS
3318-61-4
化学式
C11H12O2
mdl
MFCD00048231
分子量
176.215
InChiKey
NROOHYGFTHTDFF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:150-153℃
-
密度:1.061±0.06 g/cm3 (20 ºC 760 Torr)
-
闪点:103.2±17.4℃
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.272
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2914399090
-
储存条件:2-8°C
SDS
| Name: | 1-Phenyl-2 4-pentanedione 96% (gc) Material Safety Data Sheet |
| Synonym: | |
| CAS: | 3318-61-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3318-61-4 | 2,4-pentanedione, 1-phenyl- | 96.0 | 222-015-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 3318-61-4: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 144 deg C @ 15.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.0540g/cm3
Molecular Formula: C11H12O2
Molecular Weight: 176.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3318-61-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,4-pentanedione, 1-phenyl- - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 3318-61-4: No information available.
Canada
CAS# 3318-61-4 is listed on Canada's NDSL List.
CAS# 3318-61-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3318-61-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-苯基-2-丁酮 benzyl ethyl ketone 1007-32-5 C10H12O 148.205 —— 4-hydroxy-1-phenylpentan-2-one 174416-52-5 C11H14O2 178.231 苯基丙酮 1-phenyl-acetone 103-79-7 C9H10O 134.178 3-苯基己烷-2,4-二酮 3-phenyl-2,4-hexanedione 109839-21-6 C12H14O2 190.242 4-羟基-5-苯基戊-2-酮 4-hydroxy-5-phenylpentan-2-one 646062-85-3 C11H14O2 178.231 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯基丙酮 1-phenyl-acetone 103-79-7 C9H10O 134.178 —— 2-Pentanone, 4-hydroxy-4-methyl-1,5-diphenyl- 53189-96-1 C18H20O2 268.356
反应信息
-
作为反应物:描述:参考文献:名称:The Reaction of Sulfamide with α- and β-Diketones. The Preparation of 1,2,5-Thiadiazole 1,1-Dioxides and 1,2,6-Thiadiazine 1,1-Dioxides摘要:DOI:10.1021/jo01030a059
-
作为产物:描述:5-苯基-3-戊烯-2-酮 在 甲醇 、 水 、 氧气 、 对甲苯磺酸 、 palladium dichloride 作用下, 以 N,N-二甲基乙酰胺 为溶剂, 80.0 ℃ 、607.99 kPa 条件下, 反应 40.0h, 以64%的产率得到1-苯基-2,4-戊二酮参考文献:名称:使用钯催化剂将电子不足的内部烯烃高效原子氧化为酮摘要:使用O 2作为“绿色”氧化剂,可以从缺电子的内烯烃实现100%原子效率的酮合成(请参见方案,DMA = N,N-二甲基乙酰胺,EWG =吸电子基团)。各种电子不足的烯烃以超过99%的选择性被氧化成相应的酮,而没有形成烯烃异构体或它们的氧化产物。DOI:10.1002/anie.201301611
-
作为试剂:描述:3-((E)-3-苯基-2-丙烯酰基)-1,3-恶唑烷-2-酮 、 环戊二烯 在 1,2,2,6,6-五甲基哌啶 、 S-1,1'-联-2-萘酚 、 1-苯基-2,4-戊二酮 、 thulium(III) trifluoromethanesulfonate 作用下, 生成 3-[(1S,2R,3R,4R)-3-phenylbicyclo[2.2.1]hept-5-en-2-ylcarbonyl]-1,3-oxazolidin-2-one 、 exo-3-(3-phenylbicyclo[2.2.1]hept-5-ene-2-carbonyl)oxazolidin-2-one 、 3-(((1'R,2'S,3'S,4'S)-3'-phenylbicyclo<2.2.1>hept-5'-en-2'-yl)carbonyl)-1,3-oxazolidin-2-one参考文献:名称:手性镧系元素三氟甲烷磺酸盐催化不对称Diels-Alder反应。使用单一手性来源和非手性配体的选择,两种对映体的三氟甲磺酸酯的独特结构和立体选择性合成摘要:开发了手性镧系三氟甲烷磺酸盐(三氟甲磺酸盐),并揭示了三氟甲磺酸盐的独特结构。在催化量的三氟甲磺酸酯存在下,酰基1,3-恶唑烷-2-酮与环戊二烯反应,以高收率和高对映体过量得到狄尔斯-阿尔德加合物。根据反应,通过使用单一手性来源,R-(+)-联萘酚和选择的非手性配体,立体选择性地制备了Diels-Alder加合物的两种对映体。当3-乙酰基-1,3-恶唑烷-2-酮与由三氟甲磺酸镧系元素,R-(+)-联萘酚和顺式1,2,6-三甲基哌啶组成的原始催化剂体系结合使用时,产生了一种新的催化剂(催化剂A)。在这种催化剂3-酰基-1的存在下,3-恶唑烷-2-酮与环戊二烯反应,以过量的对映体形式提供内加合物。产品的绝对配置为2S,3R。另一方面,当将3-苯基乙酰丙酮(PAA)与原始催化剂体系(催化剂B)混合时,观察到对映体的选择性,并以过量的对映体获得了具有绝对构型2R,3S的内加合物。DOI:10.1016/s0040-4020(01)85657-x
文献信息
-
6-N-Linked Heterocycle-Substituted 2,3,4,5-Tetrahydro-1H-Benzo[d]Azepines as 5-Ht2c Receptor Agonists申请人:Briner Karin公开号:US20080214520A1公开(公告)日:2008-09-04The present invention provides 6-substituted 2,3,4,5-tetrahydro-1H-benzo[d]azepines of Formula I as selective 5-HT 2C receptor agonists for the treatment of 5-HT 2C associated disorders including obesity, obsessive/compulsive disorder, depression, and anxiety: Formula (I) where: R 6 is selected from the group consisting of (a, b, c, d, e) and other substituents are as defined in the specification.本发明提供了Formula I的6-取代的2,3,4,5-四氢-1H-苯并[d]氮杂环庚烯作为选择性5-HT2C受体激动剂,用于治疗与5-HT2C相关的疾病,包括肥胖症、强迫症、抑郁症和焦虑症:Formula (I)其中:R6选自(a、b、c、d、e)等基团组成的群,其他取代基如规范中定义。
-
Photosensitive compound申请人:Wako Pure Chemical Industries, Ltd.公开号:US05250669A1公开(公告)日:1993-10-05Photosensitive compounds having preferably a functional group such as --SO.sub.2 Cl, --SO.sub.3 H, --SO.sub.3 R, ##STR1## (R, R', R" being alkyl) on a terminal benzene or naphthalene ring connected via a methylene group and ##STR2## moiety are improved in sensitivity to light and thermal stability, and thus useful in a photo resist.具有偏好功能基团的光敏化合物,例如--SO.sub.2 Cl,--SO.sub.3 H,--SO.sub.3 R,##STR1##(R,R',R"为烷基)连接通过亚甲基基团的末端苯或萘环和##STR2##基团,在光敏度和热稳定性方面得到改善,因此在光刻胶中有用。
-
Synthesis and Antitumor Activity of Novel Pyrimidinyl Pyrazole Derivatives.作者:Hiroyuki NAITO、Masamichi SUGIMORI、Ikuo MITSUI、Yoshihide NAKAMURA、Michio IWAHANA、Mineko ISHII、Kenji HIROTANI、Eiji KUMAZAWA、Akio EJIMADOI:10.1248/cpb.47.1679日期:——Novel pyrimidinyl pyrazole derivatives were synthesized and examined for cytotoxic and antitumor activity. Mannich reaction was employed to construct this scaffold. Among the compounds synthesized, a series of propene derivatives exhibited a potent cytotoxic activity against some tumor cell lines including multidrug resistant cell lines due to the overexpression of P-glycoprotein. The vinyl bond moiety in the scaffold was believed to be required for the cytotoxic activity. Among them, compound 14g, when administered intraperitoneally, showed potent antitumor activity against the malignant ascites caused by intraperitoneal inoculation of P388 cells in mice. This compound also showed high activity against a solid tumor Meth A mouse fibrosarcoma when administered both intraperitoneally and orally.
-
Arylsulfonylcarbamoyl-1,3-dicarbonylacyclic compounds申请人:Mead Johnson & Company公开号:US03998879A1公开(公告)日:1976-12-21A new class of arylsulfonylcarbamoyl-1,3-cyclohexanediones and arylsulfonylcarbamoyl-1,3-dicarbonylacyclic compounds are disclosed. These substances have hypoglycemic or hyperglycemic properties and are of value in controlling abnormal blood sugar levels. The arylsulfonylcarbamoyl derivatives of 1,3-dicarbonylalicyclic and acyclic compounds are prepared by reacting an arylsulfonylisocyanate with a 1,3-dicarbonylalicyclic or acyclic reactant. Typical embodiments are 2-(N-p-chlorobenzenesulfonylcarbamoyl)-5,5-dimethylcyclohexane-1,3-dione and 5-(1-ethylpropyl)-2-(N-p-chlorobenzenesulfonylcarbamoyl)-1,3-cyclohexanedi one.
-
[EN] AZAINDAZOLES<br/>[FR] AZAINDAZOLES申请人:GLAX0SMITHKLINE LLC公开号:WO2013039988A1公开(公告)日:2013-03-21Herein are disclosed azaindazoles of formula (I), (I), where the various groups are defined herein, and which are useful for treating cancer.这里公开了公式(I)、(I)的氮杂吲唑,其中各团簇在本发明中定义,并且用于治疗癌症。
表征谱图
-
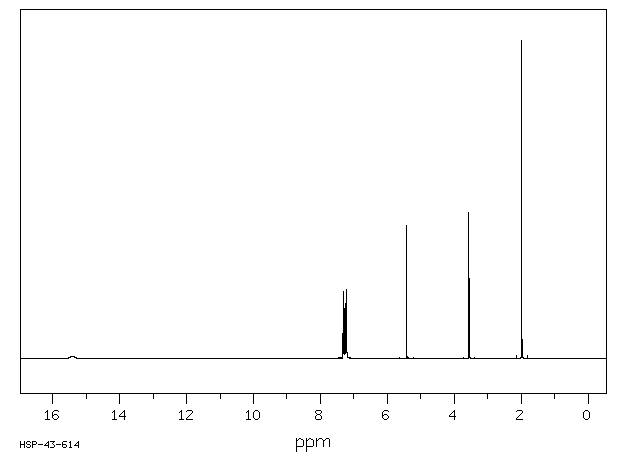
氢谱1HNMR
-
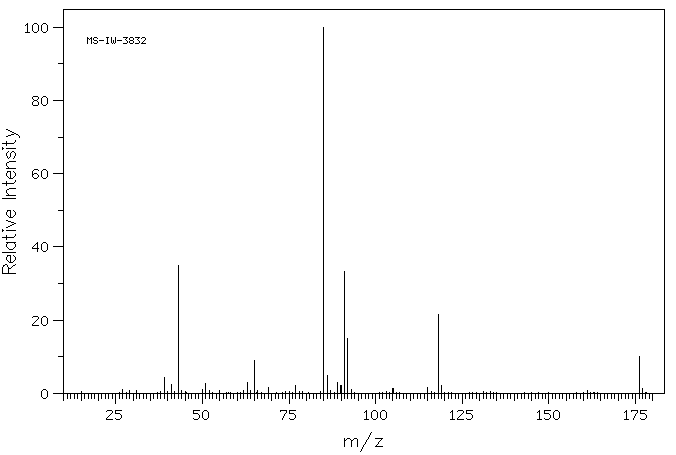
质谱MS
-
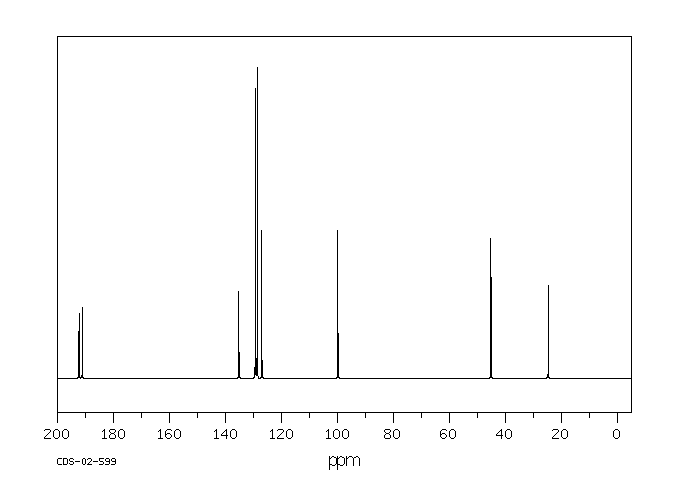
碳谱13CNMR
-
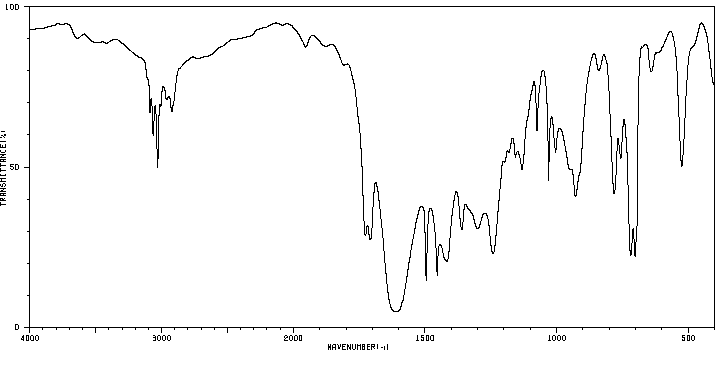
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷