甲基-β-D-吡喃木糖苷 | 612-05-5
中文名称
甲基-β-D-吡喃木糖苷
中文别名
甲基-BETA-吡喃木糖苷;甲基-β-吡喃木糖苷;甲基-beta-d-木吡喃糖苷
英文名称
methyl-β-D-xylopyranoside
英文别名
1-O-β-methyl-D-xylopyranoside;1-O-methyl-β-D-xylopyranoside;Methyl β-D-xylopyranoside;methyl-βD-xylopyranoside;1-O-methyl-β-D-xyloside;Methyl beta-D-xylopyranoside;(2R,3R,4S,5R)-2-methoxyoxane-3,4,5-triol
CAS
612-05-5
化学式
C6H12O5
mdl
MFCD00047532
分子量
164.158
InChiKey
ZBDGHWFPLXXWRD-JGWLITMVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:155-158 °C(lit.)
-
沸点:314.0±42.0 °C(Predicted)
-
密度:1.40 g/cm3
计算性质
-
辛醇/水分配系数(LogP):-2
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:79.2
-
氢给体数:3
-
氢受体数:5
安全信息
-
TSCA:Yes
-
WGK Germany:3
-
海关编码:29400090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 D-吡喃木糖 D-Xylose 10257-31-5 C5H10O5 150.131 D-木糖 D-xylose 6763-34-4 C5H10O5 150.131 beta-D-吡喃木糖 β-D-xylopyranoside 2460-44-8 C5H10O5 150.131 甲基 alpha-D-呋喃木糖苷 methyl α-D-xylofuranoside 1824-96-0 C6H12O5 164.158 —— D-(+)-xylobiose 6860-47-5 C10H18O9 282.248 苯并[pqr]四苯-12-胺 methyl 2,4-di-O-acetyl-β-D-xylopyranoside 74162-08-6 C10H16O7 248.233 —— methyl 2,3-di-O-acetyl-β-D-xylopyranoside 70003-50-8 C10H16O7 248.233 —— methyl 3,4-di-O-acetyl-β-D-xylopyranoside 77217-82-4 C10H16O7 248.233 —— methyl β-D-erythro-pentopyranosid-3-ulose 7045-35-4 C6H10O5 162.142 —— tetra-O-acetyl-β-D-xylopyranose 4049-33-6 C13H18O9 318.281 —— (1S,3R,4R,6R,7S,10R)-7-Allyloxy-10-hydroxy-3,4-dimethoxy-3,4-dimethyl-2,5,8-trioxabicyclo[4.4.0]decane 201043-05-2 C14H24O7 304.34 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (2R,3r,4r,5r)-2-甲氧基四氢-2H-吡喃-3,4,5-三醇 methyl β-D-ribopyranoside 91-09-8 C6H12O5 164.158 —— Ethyl-β-D-xylopyranosid 6743-62-0 C7H14O5 178.185 —— 3-O-methyl-β-methyl-D-xylopyranoside 7381-10-4 C7H14O5 178.185 —— Methyl 2-O-methyl-β-D-xylopyranoside 7381-12-6 C7H14O5 178.185 —— Methyl 4-O-methyl-β-D-xylopyranoside 58918-59-5 C7H14O5 178.185 D-吡喃木糖 D-Xylose 10257-31-5 C5H10O5 150.131 —— methyl O2,O4-dimethyl-β-D-xylopyranoside 51754-98-4 C8H16O5 192.212 —— methyl β-D-xylopyranoside 3-methoxymethyl ether 100663-05-6 C8H16O6 208.211 —— methyl 2,3,4-tri-O-methyl-β-D-xylopyranoside 2876-85-9 C9H18O5 206.239 —— methyl β-D-xylopyranoside 4-methoxymethyl ether 100663-06-7 C8H16O6 208.211 —— methyl β-D-xylopyranoside 2-methoxymethyl ether 100663-03-4 C8H16O6 208.211 —— methyl β-D-galactopyranosyl-(1->4)-β-D-xylopyranoside 97753-81-6 C12H22O10 326.301 —— methyl 3-O-benzyl-β-D-xylopyranoside 72521-39-2 C13H18O5 254.283 —— methyl 2,3-di-O-acetyl-β-D-xylopyranoside 70003-50-8 C10H16O7 248.233 —— methyl 3,4-di-O-acetyl-β-D-xylopyranoside 77217-82-4 C10H16O7 248.233 —— methyl β-D-erythro-pentopyranosid-3-ulose 7045-35-4 C6H10O5 162.142 —— methyl α-D-xylopyranoside 4-benzyl ether 69984-20-9 C13H18O5 254.283 —— methyl 2-O-benzyl-β-D-xylopyranoside 51755-03-4 C13H18O5 254.283 —— methyl 2,3,4-tris(O-trimethylsilyl)-β-D-xylopyranoside 18082-67-2 C15H36O5Si3 380.704 —— methyl 2,4-di-O-benzyl-β-D-xylopyranoside 80973-56-4 C20H24O5 344.408 —— methyl 3,4-di-O-benzyl-β-D-xylopyranoside 80973-55-3 C20H24O5 344.408 —— methyl 4-O-sulfo-β-D-xylopyranoside 1112527-75-9 C6H12O8S 244.222 —— 1-O-methyl-2,3,4-O-pivaloyl-β-D-xylopyranoside —— C21H36O8 416.512 —— methyl 2,3,4-tri-O-benzyl-β-D-xylopyranoside 20787-15-9 C27H30O5 434.532 - 1
- 2
- 3
反应信息
-
作为反应物:描述:甲基-β-D-吡喃木糖苷 在 甲醇 、 对甲苯磺酸 、 三氟乙酸 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 12.17h, 生成 methyl 2,3-O-isopropylidene-β-D-xylopyranoside参考文献:名称:Regioselective protection strategies for D-xylopyranosides摘要:The acylation of D-xylopyranosides can be effected at any position by selective hydroxyl activation with dibutyltin oxide in refluxing benzene and proper choice of starting anomer. Methyl 4-O-benzyl-beta-D-xylopyranoside, available from methyl 2,3-O-isopropylidene-beta-D-xylopyranoside, provides the 2- and 3-benzoates, which are easily separable in 85% combined yield. Methyl and allyl beta-D-xylopyranosides, when treated with 1 equiv of dibutyltin and subsequently with benzoyl chloride (1 equiv), yield their corresponding 4-benzoates (80%). The use of 2 equiv of benzoyl chloride provides the 3,4-dibenzoates in excellent yield (90%). The clean conversion to mono- or dibenzoates, depending on the amount of benzoyl chloride added, suggests that the intermediate stannylene acetals provide different activation levels. A pathway involving acylation of an intermediate dibutylchlorostannyl ether is proposed to explain the observed phenomenon. This sequential selective activation is used to afford protection and differentiation of the 3- and 4-positions with a one-pot synthesis of methyl 4-O-benzoyl-3-O-(chloroacetyl)-beta-D-xylopyranoside. Methyl and benzyl alpha-D-xylopyranosides afford the 2,4-dibenzoates in good yield (> 80%) demonstrating 1,3-activation of a triol system. This protection strategy is used to prepare benzyl O-(2,3,5-tri-O-benzoyl-alpha-L-arabinofuranosyl)-(1 --> 3)-2,4-di-O-benzoyl-alpha-D-xylopyranoside from D-xylose and L-arabinose. In the final step, the silver triflate catalyzed glycosylation of benzyl 2,4-di-O-benzoyl-alpha-D-xylopyranoside by 2,3,5-tri-O-benzoyl-alpha-L-arabinofuranosyl chloride is accomplished in 91% yield.DOI:10.1021/jo00025a013
-
作为产物:描述:参考文献:名称:Mosher MPTA-酯方法学对单糖的适用性摘要:石竹糖1是一种新型的12碳4 C支链单糖,是假单胞菌拟细菌的脂多糖组分中发现的多糖链的组成部分。1通过将激子手性偶联方法应用于NaIO4得到的两个片段,阐明了其绝对立体化学。多糖链的氧化。手性仲醇的绝对构型可以通过Mosher方法进行定义。3它分析了在(S)-和(R)-α-甲氧基-中邻位质子向手性中心化学位移之间差异的征兆。由该化合物获得的α-三氟甲基苯乙酸酯(MPTA)酯。但是,当将Mosher酯方法应用于石竹纤维双双亚丙基衍生物2时,无法给出完全可靠的结果。实际上,DOI:10.1080/07328309808007469
文献信息
-
Selective C−O Bond Cleavage of Sugars with Hydrosilanes Catalyzed by Piers’ Borane Generated In Situ作者:Jianbo Zhang、Sehoon Park、Sukbok ChangDOI:10.1002/anie.201708109日期:2017.10.23[(C6F5)2BH], generated in situ, is demonstrated to promote the hydrosilylative reduction of sugars, providing a series of linear or cyclic polyols with high chemo- and regioselectivities under mild conditions. Studies of catalytic reactivity and regioselectivity with regard to the C−O bond cleavage with hydrosilanes suggest an importance of the steric environment around the anomeric carbon center of the
-
Regioselective (phenylcarbamoyl)ation of polyhydroxy compounds by phenyl isocyanate-zinc naphthenate作者:Shigeyoshi Nishino、Yoshiharu IshidoDOI:10.1016/s0008-6215(00)90142-3日期:1986.11In the presence of zinc naphthenate as the catalyst, the reaction of phenyl isocyanate with 1,2-propanediol and 3,4-O-isopropylidene-d-mannitol gave the primary phenylcarbamates in high yield. 1,2,6-Hexanetriol was selectively phenylcarbamoylated at O-1, and N6-benzyladenosine at O-2′. The common methyl aldohexo- and aldopento-pyranosides gave the 3-mono(phenylcarbamate)s in fair to excellent yields
-
Process of producing 8A- and 9A-azalide antibiotics申请人:Merck & Co., Inc.公开号:US05332807A1公开(公告)日:1994-07-26A process of producing 8a- and 9a- azalide compounds is disclosed, comprised of reacting an 8a- aza or 9a- aza azalide eastern fragment or a derivative thereof with a compound of the formula: X--A'--Y wherein X and Y are appropriate reactive groups and A' is a fragment or compound which forms the western portion of the azalide, and cyclizing this intermediate to form the target 8a- or 9a-azalide compound. Compounds of formula I, II and III as well as other azalides can be synthesized according to this process.
-
Scope and limitations of carbohydrate hydrolysis for de novo glycan sequencing using a hydrogen peroxide/metallopeptide-based glycosidase mimetic作者:Tianyuan Peng、Zachary Wooke、Nicola L.B. PohlDOI:10.1016/j.carres.2018.01.008日期:2018.3conditions from analyte to analyte, greatly limits its broad utility. Herein we report studies on a hydrogen peroxide/CuGGH metallopeptide-based glycosidase mimetic design for a more efficient and controllable carbohydrate hydrolysis. A library of methyl glycosides consisting of ten common monosaccharide substrates, along with oligosaccharide substrates, was screened with the artificial glycosidase for hydrolytic
-
Simultaneous Conversion of C <sub>5</sub> and C <sub>6</sub> Sugars into Methyl Levulinate with the Addition of 1,3,5‐Trioxane作者:Xilei Lyu、Zihao Zhang、Francis Okejiri、Hao Chen、Mai Xu、Xujie Chen、Shuguang Deng、Xiuyang LuDOI:10.1002/cssc.201902096日期:2019.10.8The simultaneous conversion of C5 and C6 mixed sugars into methyl levulinate (MLE) has emerged as a versatile strategy to eliminate costly separation steps. However, the traditional upgrading of C5 sugars into MLE is very complex as it requires both acid-catalyzed and hydrogenation processes. This study concerns the development of a one-pot, hydrogenation-free conversion of C5 sugars into MLE over different将C5和C6混合糖同时转化为乙酰丙酸甲酯(MLE)已成为一种消除昂贵分离步骤的通用策略。但是,传统的将C5糖升级为MLE的过程非常复杂,因为它需要酸催化过程和加氢过程。这项研究涉及在接近关键的甲醇条件下借助1,3,5-三恶烷在不同的酸催化剂上将一锅一锅的无加氢的C5糖转化为MLE的进展。对于不添加1,3,5-三恶烷的沸石上的C5糖转化而言,由于氢化活性低,MLE收率非常低。1,3,5-三恶烷的添加通过提供不包括氢化步骤的替代转化途径,显着提高了MLE收率。直接比较五种不同沸石的催化性能表明,具有高路易斯酸位和布朗斯台德酸位密度的Hβ沸石可提供最高的MLE收率。加入1,3,5-三恶烷,糠醛衍生物和甲醛的羟甲基化是关键步骤。值得注意的是,当完全优化反应条件时,由Hβ沸石催化的C5和C6糖的同时转化可以实现高达50.4%的MLE收率。而且,Hβ沸石催化剂可以重复使用至少五次,而性能没有显着变化。
表征谱图
-
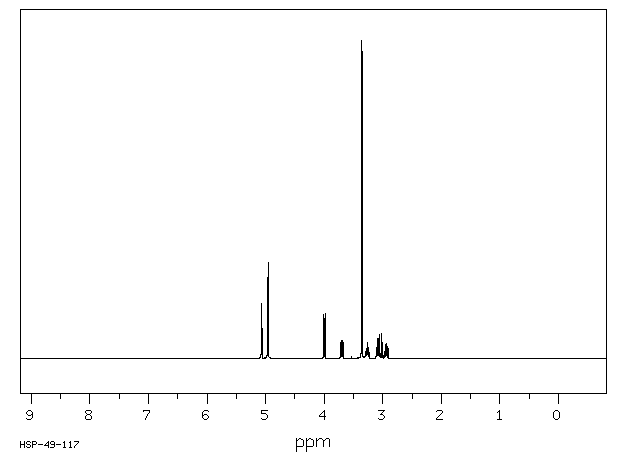
氢谱1HNMR
-
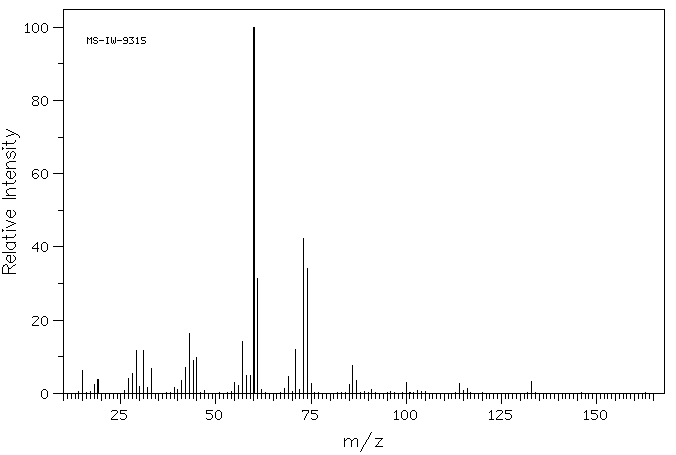
质谱MS
-
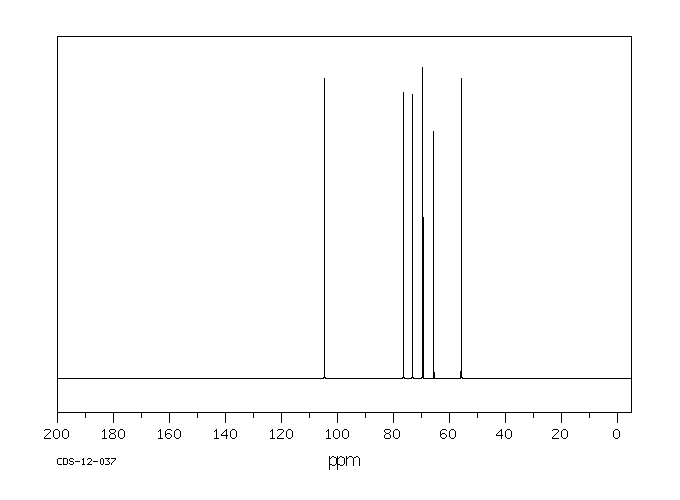
碳谱13CNMR
-
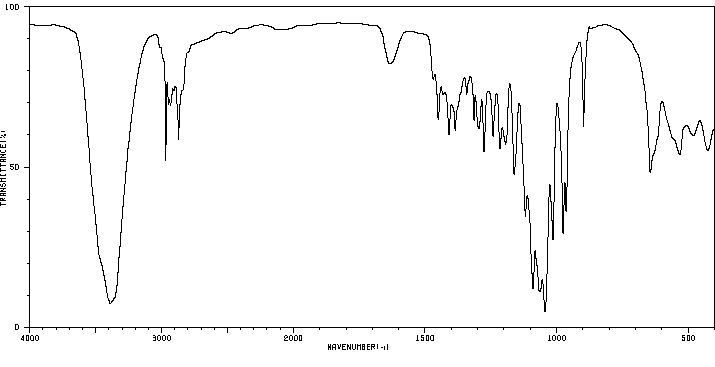
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷