胡麻黄素; 5,6,3',4'-四羟基-7-甲氧基黄酮 | 22384-63-0
中文名称
胡麻黄素; 5,6,3',4'-四羟基-7-甲氧基黄酮
中文别名
5,6,3',4'-四羟基-7-甲氧基黄酮;胡麻黄素;5,6,3',4'-四羟基-7-甲氧基黄酮
英文名称
5,6,3',4'-tetrahydroxy-7-methoxyflavone
英文别名
3',4',5,6-tetrahydroxy-7-methoxyflavone;6-hydroxyluteolin 7-methyl ether;pedalitin;flaxin;2-(3,4-dihydroxy-phenyl)-5,6-dihydroxy-7-methoxy-chromen-4-one;2-(3,4-dihydroxyphenyl)-5,6-dihydroxy-7-methoxychromen-4-one
CAS
22384-63-0
化学式
C16H12O7
mdl
——
分子量
316.267
InChiKey
QWUHUBDKQQPMQG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
溶解度:溶于氯仿、二氯甲烷、乙酸乙酯、DMSO、丙酮等。
-
物理描述:Solid
-
熔点:300-301°C
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:23
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:116
-
氢给体数:4
-
氢受体数:7
安全信息
-
海关编码:2914509090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— pedalitin 5-methyl ether 25782-18-7 C17H14O7 330.294 —— 6-hydroxyluteolin 5,7,4'-trimethyl ether 25782-20-1 C18H16O7 344.321 —— 6-hydroxyluteolin 5,7,3'-trimethyl ether 25782-21-2 C18H16O7 344.321 —— 4'-hydroxy-5,6,7,3'-tetramethoxyflavone 51145-80-3 C19H18O7 358.348 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 胡麻素四乙酸酯 Pedalitintetraacetat 25782-30-3 C24H20O11 484.416 —— 3',4'-diphenylmethylenedioxy-5,6-dihydroxy-7-methoxyflavone 104480-95-7 C29H20O7 480.474 —— 5-benzyloxy-3',4'-diphenylmethylenedioxy-6-hydroxy-7-methoxyflavone 104480-98-0 C36H26O7 570.598 —— 6-acetoxy-3',4'-diphenylmethylenedioxy-5-hydroxy-7-methoxyflavone 104480-96-8 C31H22O8 522.511 —— 3',4':5,6-bis(diphenylmethylenedioxy)-7-methoxyflavone 104480-94-6 C42H28O7 644.68
反应信息
-
作为反应物:描述:胡麻黄素; 5,6,3',4'-四羟基-7-甲氧基黄酮 在 氢氧化钾 、 potassium carbonate 、 potassium iodide 作用下, 以 吡啶 、 甲醇 、 水 、 溶剂黄146 、 丙酮 为溶剂, 反应 5.78h, 生成 5-benzyloxy-3',4'-diphenylmethylenedioxy-6-hydroxy-7-methoxyflavone参考文献:名称:Studies of the selective O-alkylation and dealkylation of flavonoids. VIII. Synthesis of pedaliin.摘要:3', 4'-双(苄氧基)-6-羟基-5, 7-二甲氧基黄酮(15)是由6-羟基-2, 4-二甲氧基-3-(甲氧基甲氧基)苯乙酮(13)通过6'-羟基得到-2', 4'-二甲氧基-3'-甲氧基-甲氧基-2-[3, 4-双(苄氧基)苯甲酰基]苯乙酮(14)。黄酮(15)的乙酸酯(16)的5-甲氧基用约5%(w/v)无水氯化铝的乙腈溶液选择性裂解,得到6-乙酰氧基-3',4'-联(苄氧基) -5-羟基-7-甲氧基黄酮(17)。通过苄基化和水解将5-羟基黄酮(17)转化为3',4',5-三(苄氧基)-6-羟基-8-甲氧基黄酮(20)。 6-羟基黄酮(20)与2,3,4,6-四-O-乙酰基-α-D-葡萄糖基溴缩合,然后水解所得化合物,得到相应的6-O-β-D-葡萄糖苷(22),通过氢解转化为3',4',5,6-四羟基-7-甲氧基黄酮6-O-β-D-葡萄糖苷(pedaliin)(1)。该方法应可作为合成 5, 6-二羟基-7-甲氧基黄酮的 6-O-葡萄糖苷的通用方法。DOI:10.1248/cpb.34.30
-
作为产物:描述:参考文献:名称:5,6,7,3′,4′-单甲氧基四羟基黄酮的合成研究:Pedalitin 的合成摘要:在针对 5,6,7,3′,4′-单甲氧基四羟基黄酮的合成研究中,实现了一种简洁的 pedalitin 合成程序。如前所述,6-羟基-2,3,4-三甲氧基苯乙酮是通过 1,4-二羟基-2,6-二甲氧基苯与三氟化硼醚酸二乙酯在乙酸中酰化制备的。当在碱性条件下进行 6-羟基-2,3,4-三甲氧基苯乙酮 2b 与香兰素的羟醛缩合时,它产生了 2′-羟基查尔酮 3b,令人惊讶的是,它与 3-羟基黄酮 4 一起产生了相当数量的 3-羟基黄酮 4。我们提出这种氧化环化可能是由于醌化物的贡献,可能会受到有氧氧化。然后将查尔酮与碘在二甲基亚砜中进行氧化环化,得到产量良好的黄酮 5。令我们高兴的是,在溴化氢溶液(30% 的乙酸溶液)下,3 个 5-、6-和 3′-位置的三个甲氧基的连续去甲基化顺利进行,产生 pedalitin 1。3-羟基黄酮 4 和 pedalitin tetraacetate 6 的晶体结构通过DOI:10.3390/molecules29020513
文献信息
-
Compositions of flavonoids and flavonoid-containing extracts and the treatment of diseases申请人:Gong Qiang Bang公开号:US20050049206A1公开(公告)日:2005-03-03In this invention, we describe a group of flavonoids and flavonoid-containing extracts that have pharmaceutical properties which are useful in the medicinal therapy of fibrotic diseases for the treatment or reparation and prevention of fibrotic lesional tissues. Representative flavonoids and flavonoid-containing extracts have the active compositions of the below formula. Those compositions can be extracted and purified from the botanicals, including Scutellaria baicalensis Georgi, Scutellaria scordifolia Fisch,Oroxylum indicum(L.) Vent, Plantago major L. The compositions of the invention are novel as an anti-fibrotic drugs, as agents for treating fibrosis.
-
Flavonoids of Teucrium hircanicum作者:G. B. Oganesyan、V. A. MnatsakanyanDOI:10.1007/bf00596665日期:1987.11
-
Studies of the selective O-alkylation and dealkylation of flavonoids. 13. An improved method for synthesizing 5,6,7-trihydroxyflavones from 6-hydroxy-5,7-dimethoxyflavones作者:Tokunaru Horie、Hideaki Tominaga、Yasuhiko Kawamura、Toshihide YamadaDOI:10.1021/jo00038a023日期:1992.6The demethylation of five 6-hydroxy-5,7-dimethoxyflavones 1 and their acetates with 30% w/v anhydrous aluminum chloride in acetonitrile was studied, and the following results were found. In the demethylation of 6-hydroxy-4',5,7-trimethoxyflavone (1a), 5,6-dihydroxy-4',7-dimethoxyflavone (2a) and 5,6,7-trihydroxy-4'-methoxyflavone (3a) were produced. Although the ratio of the two products varied according to the amount of aluminum chloride used, it became constant after 12-24 h because the cleavage of the 7-methoxy group in 2a was suppressed by iminoesterification of the 6-hydroxy group. In contrast, the demethylation of the 5- and 7-methoxy groups of acetate 4a proceeded smoothly by the process shown in Scheme II. The amount of 3a increased with increasing reaction time to give 3a as the main product after 36-48 h. The same phenomena were observed in the demethylation of the other 6-hydroxyflavones 1b-1e and their acetates 4b-4e. The demethylation of the acetates is widely applicable as a general method for synthesizing 5,6,7-trihydroxyflavones because the protection of hydroxy groups also suppresses the further cleavage of the methoxy group adjacent to the acetoxy group on the B ring of compounds such as 4d and 4e.
-
OGANESYAN, G. B.;MNATSAKANYAN, V. A., XIMIYA PRIROD. SOED.,(1987) N 6, 910-911作者:OGANESYAN, G. B.、MNATSAKANYAN, V. A.DOI:——日期:——
-
HORIE TOKUHARU, NIXON KAGAKU KAJSI, NIRRON KAGAKU KAISNI, J. CHEM. SOS. JAR., CHEM. AND I+作者:HORIE TOKUHARUDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
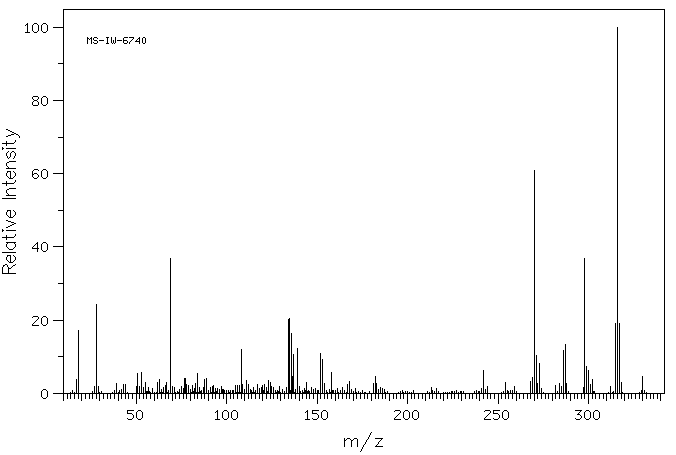
质谱MS
-
碳谱13CNMR
-
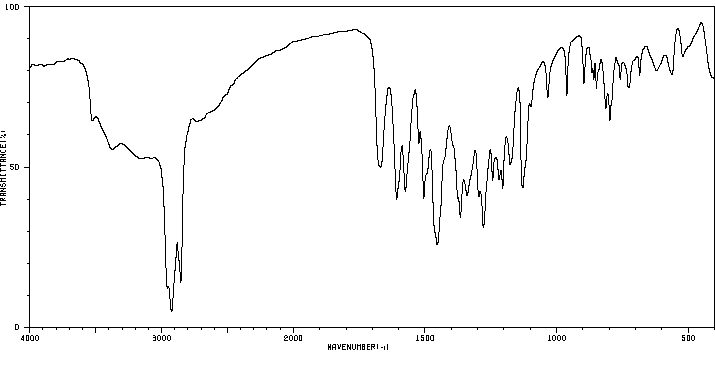
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷