香豆素 151 | 53518-15-3
中文名称
香豆素 151
中文别名
香豆素490;7-氨基-4-氟甲醇;7-氨基-4-三氟甲基香豆素;香豆素151;7-氨基-4-(三氟甲基)香豆素
英文名称
Coumarin 151
英文别名
7-amino-4-(trifluoromethyl)coumarin;AFC;7-amino-4-(trifluoromethyl)chromen-2-one
CAS
53518-15-3
化学式
C10H6F3NO2
mdl
MFCD00006858
分子量
229.158
InChiKey
JBNOVHJXQSHGRL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:221-222 °C(lit.)
-
沸点:314.6±42.0 °C(Predicted)
-
密度:1.4096 (estimate)
-
稳定性/保质期:
如果按照规定使用和储存,就不会分解,并且没有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:16
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:52.3
-
氢给体数:1
-
氢受体数:6
安全信息
-
TSCA:T
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S26,S36,S37/39
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:2932209090
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
| Name: | Coumarin 151 Laser Grade 99% (UV-Vis) Material Safety Data Sheet |
| Synonym: | 2H-1-Benzopyran-2-One,7-Amino-4-(Trifluoromethyl) |
| CAS: | 53518-15-3 |
Synonym:2H-1-Benzopyran-2-One,7-Amino-4-(Trifluoromethyl)
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 53518-15-3 | 2H-1-Benzopyran-2-One,7-Amino-4-(Trifl | 99 | 258-599-1 |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
The toxicological properties of this substance have not been fully investigated.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 53518-15-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 221 - 222 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H6F3NO2
Molecular Weight: 229.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids, strong bases.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, hydrogen fluoride gas, organic fluorides.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 53518-15-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2H-1-Benzopyran-2-One,7-Amino-4-(Trifluoromethyl) - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 53518-15-3: No information available.
Canada
CAS# 53518-15-3 is listed on Canada's NDSL List.
CAS# 53518-15-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 53518-15-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
生物活性
7-氨基-4-(三氟甲基)苯并恶唑(Coumarin 151)是一种荧光标记物,可用于灵敏检测蛋白酶。其激发和发射波长分别为400 nm 和 500 nm。
体外研究氨基酸衍生物和肽衍生物的7-氨基-4-(三氟甲基)苯并恶唑被用于监测肽酶活性。使用荧光底物 N-乙酰天冬氨酸-谷氨酰天冬氨酸-天冬氨酸-7-氨基-4-(三氟甲基)苯并恶唑(Ac-DEVD-AFC)来测量细胞凋亡蛋白酶的激活情况。该底物能够快速进入细胞,并在特定 caspases 作用下,在天冬氨酸残基处被高效水解,生成荧光化合物7-氨基-4-(三氟甲基)苯并恶唑(AFC)。随后通过细胞裂解释放出的7-氨基-4-(三氟甲基)苯并恶唑在HPLC上分离,并通过荧光检测。使用pancaspase抑制剂苯甲酰基-缬氨-天冬氨酸-丙酮酸酯对细胞内7-氨基-4-(三氟甲基)苯并恶唑的生成进行阻断,从而确认是由内源性 caspases 负责水解过程。
γ-谷氨酰-7-氨基-4-(三氟甲基)苯并恶唑被合成并用作 γ-谷氨酰转肽酶荧光测定的底物。该反应产物 7-氨基-4-(三氟甲基)苯并恶唑在中性pH值下具有荧光性,其激发和发射波长分别为400 nm 和490 nm,在10至300 pmol/3 mL 反应混合物的范围内,荧光强度与浓度呈线性关系。
用途7-Amino-4-(trifluoromethyl)coumarin 可用作激光染料和蛋白酶的荧光标记。它也适于作为激光染料使用。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 7-isocyanato-4-(trifluoromethyl)-2H-chromen-2-one 1574594-90-3 C11H4F3NO3 255.153 [2-氧代-4-(三氟甲基)-2H-1-苯并吡喃-7-基]氨基甲酸乙酯 7-(Carbethoxyamido)-4-(trifluoromethylcoumarin) 63450-46-4 C13H10F3NO4 301.222 —— 7-(2-hydroxybenzylideneamino)-4-(trifluoromethyl)-2H-chromen-2-one 309734-26-7 C17H10F3NO3 333.267 —— 7-[(2-Hydroxynaphthalen-1-yl)methylideneamino]-4-(trifluoromethyl)chromen-2-one 1398531-34-4 C21H12F3NO3 383.326 Ac-Asp-Glu-Val-Asp-7-氨基-4-三氟甲基香豆素 Ac-DEVD-AFC 201608-14-2 C30H34F3N5O13 729.621 —— [4-[(4,5-dimethoxy-2-nitrophenyl)methoxy]phenyl]methyl N-[2-oxo-4-(trifluoromethyl)chromen-7-yl]carbamate 1609986-36-8 C27H21F3N2O9 574.467 —— Ac-IETD-AFC 211990-57-7 C31H38F3N5O12 729.664 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 7-isocyanato-4-(trifluoromethyl)-2H-chromen-2-one 1574594-90-3 C11H4F3NO3 255.153 —— 7-[N-(2-bromoethyl)amino]-4-trifluoromethylcoumarin 427880-49-7 C12H9BrF3NO2 336.108 —— 7-<(Trifluoroethyl)amino>-4-(trifluoromethyl)coumarin 78277-35-7 C12H7F6NO2 311.183 —— 7-acetylamino-4-(trifluoromethyl)coumarin 78277-38-0 C12H8F3NO3 271.196 —— 7-(benzylideneamino)-4-trifluoromethyl-2H-chromen-2-one 1204655-38-8 C17H10F3NO2 317.267 —— (Z)-methyl 3-((2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl)-amino)acrylate 1476745-44-4 C14H10F3NO4 313.233 —— 7-(Trifluoroacetylamino)-4-(trifluoromethyl)-2H-1-benzopyran-2-one 1336913-80-4 C12H5F6NO3 325.167 —— 7-(2-hydroxybenzylideneamino)-4-(trifluoromethyl)-2H-chromen-2-one 309734-26-7 C17H10F3NO3 333.267 —— 7-Benzamido-4-(trifluoromethyl)coumarin 78277-39-1 C17H10F3NO3 333.267 —— 7-bromo-4-trifluoromethylcoumarin 179113-04-3 C10H4BrF3O2 293.04 —— diethyl [[(4-(trifluoromethyl)-2-oxo-2H-[1]-benzopyrano-7-yl)amino]methylene]malonate 202200-94-0 C18H16F3NO6 399.323 —— [4-[[2-Oxo-4-(trifluoromethyl)chromen-7-yl]carbamoyl]phenyl]boronic acid 859500-77-9 C17H11BF3NO5 377.084 —— 7-[N-(p-toluenesulfonyl)amino]-4-trifluoromethylcoumarin 427880-47-5 C17H12F3NO4S 383.348 —— N-(4-Trifloromethylcoumarin-7-yl)-4-chlorobenzenesulfonamide 873943-54-5 C16H9ClF3NO4S 403.766 —— 7-[2-[Bis(pyridin-2-ylmethyl)amino]ethylamino]-4-(trifluoromethyl)chromen-2-one 1432733-48-6 C24H21F3N4O2 454.452 —— Ethyl 2-[(2-ethoxy-2-oxoethyl)-[2-[2-[2-[[22-oxo-22-[[2-oxo-4-(trifluoromethyl)chromen-7-yl]amino]docosa-10,12-diynoyl]amino]ethoxy]ethoxy]ethyl]amino]acetate 287401-76-7 C46H64F3N3O10 876.023 —— N-[2-oxo-4-(trifluoromethyl)chromen-7-yl]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide 859175-95-4 C23H21BF3NO5 459.23 2-甲基-2-丙基[(2S)-6-乙酰氨基-1-氧代-1-{[2-氧代-4-(三氟甲基)-2H-苯并吡喃-7-基]氨基}-2-己烷基]氨基甲酸酯 (S)-[5-acetylamino-1-(2-oxo-4-trifluoromethyl-2H-chromen-7-ylcarbamoyl)pentyl]carbamic acid tert-butyl ester 787549-23-9 C23H28F3N3O6 499.487 —— (S)-[6-bromo-1-(2-oxo-4-trifluoromethyl-2H-chromen-7-ylcarbamoyl)hexyl]carbamic acid tert-butyl ester 908859-95-0 C22H26BrF3N2O5 535.358 —— 7-[N-(2-bromoethyl)-N-(p-toluenesulfonyl)amino]-4-trifluoro-methyl coumarin 427880-48-6 C19H15BrF3NO4S 490.298 —— 4-[[(1S)-1-[[(1S)-1-[[(1S)-1-[[(1S)-1-[(4-hydroxyphenyl)methyl]-2-oxo-2-[[2-oxo-4-(trifluoromethyl)chromen-7-yl]amino]ethyl]carbamoyl]-2-methyl-propyl]carbamoyl]-3-methyl-butyl]carbamoyl]-3-methyl-butyl]amino]-4-oxo-butanoic acid 1135689-24-5 C40H50F3N5O10 817.86 —— 4'-(4-(3,5-bis(N-(4-trifluoromethyl-7-aminocoumarin)methyl)phenoxymethyl)phenyl)-2,2':6',2"-terpyridine 571193-65-2 C50H33F6N5O5 897.833 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:Synthesis and chemistry of 7-amino-4-(trifluoromethyl)coumarin and its amino acid and peptide derivatives摘要:DOI:10.1021/jo01300a003
-
作为产物:描述:参考文献:名称:Design, synthesis, cytotoxicity and mechanism of novel dihydroartemisinin-coumarin hybrids as potential anti-cancer agents摘要:To develop novel agents with anticancer activities, thirty-four new dihydroartemisinin-coumarin hybrids were designed and synthesized in this study. Those compounds were identified that had great anticancer activity against two cancer cell lines (MDA-MB-231 and HT-29). The structure-activity relationships of the derivatives were also discussed, and the results of docking analysis had shown that carbonic anhydrases IX (CA IX) was very likely to be one of the drug targets of the hybrids. Meanwhile, to clarify the mechanism of the anticancer activity of the hybrids molecule, we did further exploration in the bioactivity of the hybrids. The results had shown that these derivatives obviously inhibited proliferation of HT-29 cell lines, arrested G(0)/G(1) phase of HT-29 cells, suppressed the migration of tumor cells, and induced a great decrease in mitochondrial membrane potential leading to apoptosis of cancer cells. Interestingly, the hybrids also induced the other cell death pathway-ferroptosis. (C) 2018 Elsevier Masson SAS. All rights reserved.DOI:10.1016/j.ejmech.2018.04.005
-
作为试剂:参考文献:名称:具有均相和非均相光催化剂的叠氮化物/氧光催化,用于无环/环状烯烃和迈克尔受体的1,2-氨基羟基化摘要:能够以低竞争单线态氧量子产率氧化叠氮化物阴离子的均相和非均相光催化剂被用于生成叠氮基自由基。这些自由基会添加到富电子的和贫电子的(迈克尔受体)烯烃中,并且碳自由基是区域选择性形成的。三重态氧的捕集(I型光氧化)是受扩散控制的,随着光催化剂的再生,最初形成的过氧自由基会减少。荧光猝灭研究表明在第一步催化步骤中快速的光诱导电子转移。双环萜烯系列(pine烯,li烯)中缺乏重排产物,这说明了随后迅速进行的氧捕获和反电子转移步骤。1,2-叠氮基氢过氧化可合成1,2-叠氮基醇和1,2-氨基醇通过不同的还原方案。底物修饰和II型光氧合与电子转移光催化的结合可以合成1-氨基-2,3-二醇和2-氨基-1,3-二醇。DOI:10.1007/s11164-012-0629-3
文献信息
-
芳基卤化物及其合成方法和应用申请人:华东师范大学公开号:CN111138307A公开(公告)日:2020-05-12
-
Thrombin inhibitors申请人:——公开号:US20020119992A1公开(公告)日:2002-08-29Compounds of the invention are useful in inhibiting thrombin and associated thrombotic occlusions having the following structure: 1 or a pharmaceutically acceptable salt thereof, e.g. where R 3 is —CH 2 NH 2 , —CH 2 CH 2 NH 2 , or —CH 2 NHC(O)OC(CH 3 ) 3 .该发明的化合物在抑制凝血酶和相关血栓闭塞方面具有以下结构: 1 或其药用可接受的盐,例如其中R 3 为—CH 2 NH 2 ,—CH 2 CH 2 NH 2 ,或—CH 2 NHC(O)OC(CH 3 ) 3 。
-
[EN] FLUORESCENT SUBSTRATES FOR POLY(ADP-RIBOSYL) HYDROLASES<br/>[FR] SUBSTRATS FLUORESCENTS POUR POLY(ADP-RIBOSYL) HYDROLASES申请人:UNIV ILLINOIS公开号:WO2020055753A1公开(公告)日:2020-03-19The post-translational modification (PTM) and signaling molecule poly(ADP-ribose) (PAR) has an impact on diverse biological processes. PTM is regulated by a series of ADP-ribosyl glycohydrolases (PARG enzymes) that cleave polymers and/or liberate monomers from their protein targets. Disclosed herein is a substrate for monitoring PARG activity, TFMU-ADPr, which directly reports on total PAR hydrolase activity via release of a fluorophore; this substrate has excellent reactivity, generality, stability, and usability. A second substrate, TFMU-IDPr, selectively reports on PARG activity only from the enzyme ARH3. Use of these probes in whole-cell lysate experiments has revealed a mechanism by which ARH3 is inhibited by cholera toxin. TFMU-ADPr and TFMU-IDPr are versatile tools for assessing small-molecule inhibitors in vitro and probing the regulation of ADP-ribosyl catabolic enzymes.
-
Investigations using fluorescent ligands to monitor platinum(iv) reduction and platinum(ii) reactions in cancer cells作者:Elizabeth J. New、Ran Duan、Jenny Z. Zhang、Trevor W. HambleyDOI:10.1039/b821603g日期:——Coordination of the aniline containing fluorophores, coumarin 120 (C120) and coumarin 151 (C151) at the non-leaving group positions of cisplatin analogues (giving cis-[PtCl2(C120)(NH3)] and cis-[PtCl2(C151)(NH3)]) resulted in partial and complete quenching of the fluorescence, respectively. Oxidation of the coumarin 120 complex to the Pt(IV) form (cis,trans,cis-[PtCl2(OH)2(C120)(NH3)]) resulted in further quenching compared to that seen for the Pt(II) complex. The fluorescence profiles of these coumarin complexes were collected to evaluate their suitability for studying the metabolism of cisplatin-based anticancer drugs. C151 has the more suitable profile with a lower energy excitation peak and a better separation between the excitation and emission spectra. The complete damping of fluorescence on coordination to Pt(II) makes it unsuitable for monitoring the reduction process, but does allow it to be used to monitor loss of the aniline type ligand. All of the coumarin complexes revealed moderate cyotoxcities in the range 10–22 μM indicating that they are suitable models of anticancer agents. DNA dampens the fluorescence of both Pt(II) complexes and that of C120 has a much higher DNA binding affinity (10 000 M−1) than does the complex of C151 (300 M−1). Treatment of A2780 human ovarian carcinoma cells with the Pt-coumarin complexes resulted in fluorescence visible by confocal microscopy, and co-localisation studies with organelle specific dyes suggest they are concentrated in the late endosomes or lysosomes. Cells treated with the Pt(IV) complex of C120 revealed strong fluorescence and a somewhat different distribution to cells treated with the Pt(II) complex indicating reduction following uptake.含有苯胺的荧光团,即香豆素120(C120)和香豆素151(C151),与顺铂类似物的不离去位点协同作用(得到cis-[PtCl2(C120)(NH3)]和cis-[PtCl2(C151)(NH3)])分别导致部分和完全荧光淬灭。将香豆素120复合物氧化为Pt(IV)形式(cis,trans,cis-[PtCl2(OH)2(C120)(NH3)])则导致进一步的淬灭,相比之下,Pt(II)复合物也有这种情况。收集了这些香豆素复合物的荧光光谱,以评估它们是否适合用于研究基于顺铂的抗癌药物的代谢。C151具有更合适的荧光光谱,其激发峰能量较低,且激发光谱和发射光谱之间有更好的分离。完全淬灭荧光的协同作用使其不适合用于监测还原过程,但可用于监测苯胺类配体的丢失。所有香豆素复合物在10-22μM的范围内显示出中等细胞毒性,表明它们是适合用于抗癌剂的模型。DNA抑制了两种Pt(II)复合物的荧光,而C120与DNA的结合亲和力(10000 M-1)远高于C151复合物(300 M-1)。使用顺铂-香豆素复合物处理人卵巢癌细胞A2780,通过共聚焦显微镜可观察到荧光,并与特定细胞器的染料共定位研究表明,它们主要集中在晚期内体或溶酶体中。与使用Pt(II)复合物处理的细胞相比,用C120的Pt(IV)复合物处理的细胞显示出强烈的荧光,且分布略有不同,表明在摄取后发生还原反应。
-
SMMR (small molecule metabolite reporters) for use as in vivo glucose biosensors申请人:Bellott M. Emile公开号:US20070110672A1公开(公告)日:2007-05-17Small Molecule Metabolite Reporters (SMMRs) for use as in vivo glucose biosensors, sensor compositions, and methods of use, are described. The SMMRs include boronic acid-containing xanthene, coumarin, carbostyril and phenalene-based small molecules which are used for monitoring glucose in vivo, advantageously on the skin.
表征谱图
-
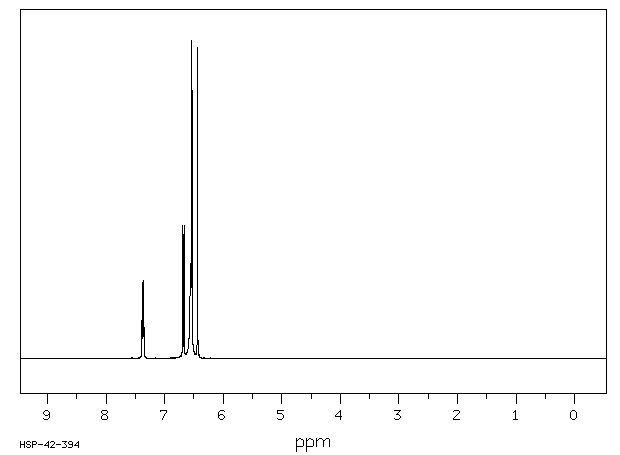
氢谱1HNMR
-
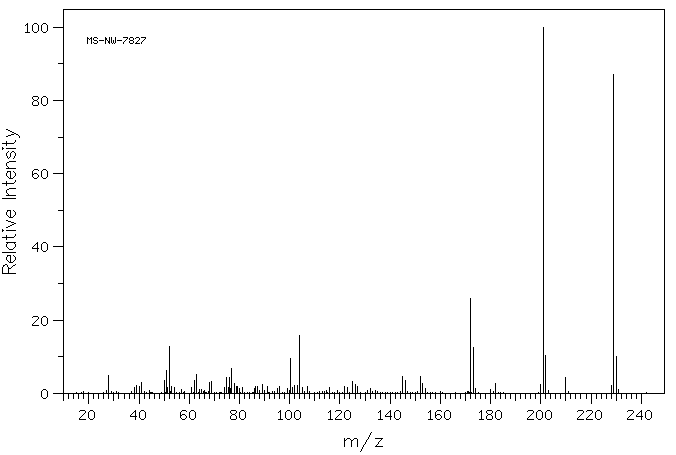
质谱MS
-
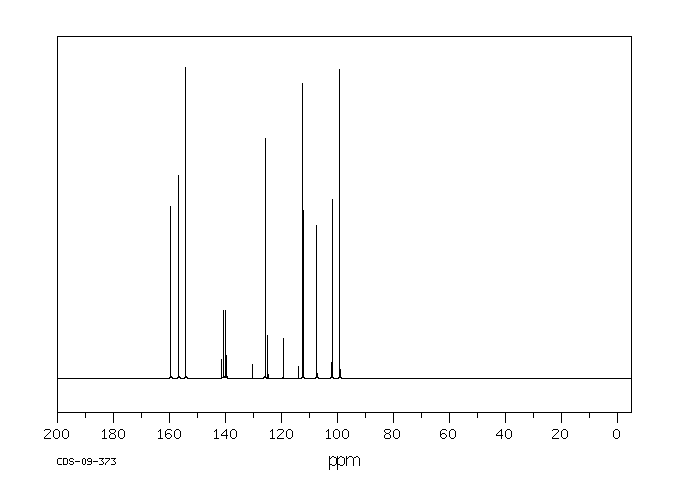
碳谱13CNMR
-
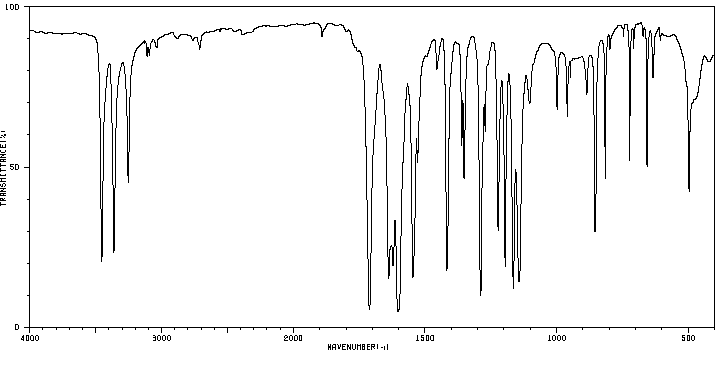
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯