5-(2-methylene-5,5,8a-trimethyl-(1R,4aR,8aR)-decahydronaphthalen-1-yl)-3-methylpent-2E-enoic acid | 20257-75-4
中文名称
——
中文别名
——
英文名称
5-(2-methylene-5,5,8a-trimethyl-(1R,4aR,8aR)-decahydronaphthalen-1-yl)-3-methylpent-2E-enoic acid
英文别名
(-)-copalic acid;ent-copalic acid;copalic acid;(E)-5-[(1R,4aR,8aR)-5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]-3-methylpent-2-enoic acid
CAS
20257-75-4
化学式
C20H32O2
mdl
——
分子量
304.473
InChiKey
JFQBNOIJWROZGE-HPMXROJMSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):6.3
-
重原子数:22
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— anticopalic acid 24470-48-2 C20H32O2 304.473 —— methyl ent-labda-8(17),13-E-dien-15-oate, methyl copalate 10395-37-6 C21H34O2 318.5 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl ent-labda-8(17),13-E-dien-15-oate, methyl copalate 10395-37-6 C21H34O2 318.5 —— (+/-)-14α-H-isogath-12-en-15-ol 82570-46-5 C20H34O 290.489
反应信息
-
作为反应物:描述:5-(2-methylene-5,5,8a-trimethyl-(1R,4aR,8aR)-decahydronaphthalen-1-yl)-3-methylpent-2E-enoic acid 在 lithium aluminium tetrahydride 、 甲酸 、 间氯过氧苯甲酸 作用下, 以 四氢呋喃 、 乙醚 、 二氯甲烷 为溶剂, 反应 46.0h, 生成 (1S,4aS,4bR,8aR,10aS)-1-Hydroxymethyl-2,4b,8,8,10a-pentamethyl-tetradecahydro-phenanthren-3-ol参考文献:名称:The C-13 configuration of the bromine-containing diterpene isoaplysin-20. Synthesis of debromoisoaplysin-20 and C-13 epimer摘要:DOI:10.1021/jo01291a028
-
作为产物:描述:穿心莲内酯 在 4-二甲氨基吡啶 、 sodium tetrahydroborate 、 potassium permanganate 、 偶氮二异丁腈 、 硫酸 、 potassium tert-butylate 、 三正丁基氢锡 、 乙酸酐 、 sodium hydride 、 magnesium sulfate 、 对甲苯磺酸 、 potassium hydroxide 、 sodium hydroxide 作用下, 以 四氢呋喃 、 乙醇 、 二氯甲烷 、 氯仿 、 水 、 二甲基亚砜 、 异丙醇 、 丙酮 、 甲苯 、 叔丁醇 为溶剂, 反应 66.01h, 生成 5-(2-methylene-5,5,8a-trimethyl-(1R,4aR,8aR)-decahydronaphthalen-1-yl)-3-methylpent-2E-enoic acid参考文献:名称:通过区域选择性的Barton-McCombie反应从穿心莲内酯合成(-)-鸟氨酸和(-)-铜酸摘要:描述了具有抗菌活性的对-拉丹二萜(-)-agathic酸(1)的首次合成。从容易获得且廉价的穿心莲内酯开始,采用具有14个步骤的线性序列的手性池方法。就Barton-McCombie自由基反应而言,区域选择性脱氧完成了合成中的关键步骤。(-)-椰油酸(2),(-)-agathic酸的类似物,已从关键中间体7分五步方便地合成。DOI:10.1016/j.tet.2015.12.022
文献信息
-
Synthetic Studies on Natural Diterpenoid Glyceryl Esters作者:Nicon Ungur、Margherita Gavagnin、Angelo Fontana、Guido CiminoDOI:10.1016/s0040-4020(00)00097-1日期:2000.4Synthesis of natural bicyclic and tricyclic diterpenoid diacylglycerols has been performed by regioselective coupling of terpenoid acid with glycerol at 1′-sn position. This method may be considered a general approach to obtain optically active acylglycerols. The preparation of 13C-labelled geranylgeranoic acid glyceryl esters is also described here.
-
Copalic acid analogs down-regulate androgen receptor and inhibit small chaperone protein作者:Nethrie D. Idippily、Qiaoyun Zheng、Chunfang Gan、Aicha Quamine、Morgan M. Ashcraft、Bo Zhong、Bin SuDOI:10.1016/j.bmcl.2017.04.046日期:2017.6Copalic acid, one of the diterpenoid acids in copaiba oil, inhibited the chaperone function of α-crystallin and heat shock protein 27kD (HSP27). It also showed potent activity in decreasing an HSP27 client protein, androgen receptor (AR), which makes it useful in prostate cancer treatment or prevention. To develop potent drug candidates to decrease the AR level in prostate cancer cells, more copalic
-
New Non-Toxic Semi-Synthetic Derivatives from Natural Diterpenes Displaying Anti-Tuberculosis Activity作者:Priscilla Matos、Brian Mahoney、Yohan Chan、David Day、Mirela Cabral、Carlos Martins、Raquel Santos、Jairo Bastos、Philip Page、Vladimir HelenoDOI:10.3390/molecules201018264日期:——We report herein the synthesis of six diterpene derivatives, three of which are new, generated through known organic chemistry reactions that allowed structural modification of the existing natural products kaurenoic acid (1) and copalic acid (2). The new compounds were fully characterized using high resolution mass spectrometry, infrared spectroscopy, 1H- and 13C-NMR experiments. We also report the evaluation of the anti-tuberculosis potential for all compounds, which showed some promising results for Micobacterium tuberculosis inhibition. Moreover, the toxicity for each of the most active compounds was also assessed.
-
Antitubercular Activity Increase in Labdane Diterpenes from Copaifera Oleoresin through Structural Modification作者:Aline Silva、Ana Carolina Soares、Mirela Cabral、Alex de Andrade、Marilza da Silva、Carlos Martins、Rodrigo Veneziani、Sérgio Ambrósio、Jairo Bastos、Vladimir HelenoDOI:10.21577/0103-5053.20160268日期:——The labdane diterpenes copalic acid, 3 beta-acetoxy-copalic acid, 3 beta-hydroxy-copalic acid and ent-agathic acid were isolated from Copaifera langsdorffii oleoresin. These four compounds were submitted to structural modifications by reduction with hydrogen/palladium, esterification with diazomethane, esterification with methanol/sulfuric acid and conversion into sodium salt, furnishing 15 compounds. All compounds were assayed in vitro against Mycobacterium tuberculosis (H37Rv, ATCC 27294). The four compounds displayed minimum inhibitory concentration (MIC) value of 125 mu g mL(-1), and were not considered active. A methylated derivative of compound 3 beta-hydroxy-copalic acid, and a sodium salt of copalic acid displayed MIC values of 25 mu g mL(-1) (71.7 mu M) and 6.25 mu g mL(-1) (19.2 mu M), respectively. The sodium salt of copalic acid stood out by displaying similar activity in comparison with streptomycin (MIC 6.25 mu g mL(-1)) and a better activity compared to mu M value of pyrazinamide (MIC 3.12 mu g mL(-1); 25.34 mu M). Therefore, the methylated derivative of compound 3 beta-hydroxy-copalic acid and the sodium salt of copalic acid should be considered for further studies.
-
(13<i>E</i>)-(−)-Labda-8(17),13-dien-15-oic Acid作者:R. Atencio、R. Gaitán、S. Pekerar、J. D. MedinaDOI:10.1107/s0108270197003016日期:1997.8.15In the title compound, C20H32O2, the two six-membered rings have a C-1(4) chair conformation and the rings are trans-fused. The crystal structure is stabilized by approximately symmetric R-2(2)(8)-type hydrogen bonds.
表征谱图
-
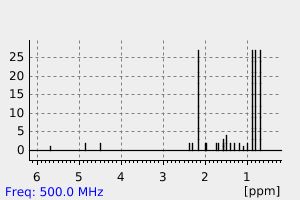
氢谱1HNMR
-
质谱MS
-
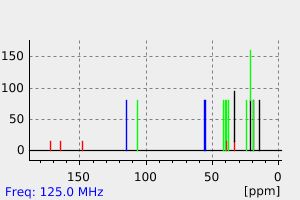
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸