3-氯-4'-甲氧基苯丙酮 | 35999-20-3
中文名称
3-氯-4'-甲氧基苯丙酮
中文别名
对甲氧基-3’-氯苯丙酮;β-氯-4-甲氧基苯丙酮;3-氯-4’-甲氧基苯丙酮;3-氯-4"-甲氧基苯丙酮;对甲氧基-3"-氯苯丙酮
英文名称
3-chloro-4'-methoxypropiophenone
英文别名
3-chloro-1-(4-methoxyphenyl)propan-1-one
CAS
35999-20-3
化学式
C10H11ClO2
mdl
MFCD00236602
分子量
198.649
InChiKey
FJBUZSAECLLZOL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:61.0 to 65.0 °C
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2914700090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温和干燥环境下使用。
SDS
3-Chloro-4'-methoxypropiophenone Revision number: 5.3
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3-Chloro-4'-methoxypropiophenone
Revision number: 5.3
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3-Chloro-4'-methoxypropiophenone
Percent: >95.0%(GC)
CAS Number: 35999-20-3
Chemical Formula: C10H11ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
3-Chloro-4'-methoxypropiophenone
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Slightly pale yellow
No data available
Odour:
3-Chloro-4'-methoxypropiophenone
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
pH: No data available
Melting point/freezing point:63°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
3-Chloro-4'-methoxypropiophenone
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3-Chloro-4'-methoxypropiophenone
Revision number: 5.3
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3-Chloro-4'-methoxypropiophenone
Percent: >95.0%(GC)
CAS Number: 35999-20-3
Chemical Formula: C10H11ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
3-Chloro-4'-methoxypropiophenone
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Slightly pale yellow
No data available
Odour:
3-Chloro-4'-methoxypropiophenone
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
pH: No data available
Melting point/freezing point:63°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
3-Chloro-4'-methoxypropiophenone
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(4-甲氧基苯基)-3-苯基-丙-1-酮 1-(4-methoxyphenyl)-3-phenylpropan-1-one 5739-38-8 C16H16O2 240.302 —— 1-(4-methoxyphenyl)-3-methoxy-1-propanone 56475-33-3 C11H14O3 194.23 1-(4-甲氧基苯基)-4-甲基戊-1-酮 1-(4-methoxyphenyl)-4-methylpentan-1-one 21550-01-6 C13H18O2 206.285 3-氯代苯丙酮 3-chloropropiophenone 936-59-4 C9H9ClO 168.623 —— 1-(4-methoxyphenyl)prop-2-en-1-one 7448-86-4 C10H10O2 162.188 —— 1- -3-anilino-propanon-(1) 19832-71-4 C16H17NO2 255.316 —— 4-(3-Morpholino-propionyl)-anisol 5770-77-4 C14H19NO3 249.31 —— (R)-3-chloro-1-(4'-methoxyphenyl)propan-1-ol 345949-40-8 C10H13ClO2 200.665 —— 3-chloro-1-(4-methoxyphenyl)propan-1-ol 50786-48-6 C10H13ClO2 200.665 1-(4-羟基苯基)丙-2-烯-1-酮 1-(4-hydroxyphenyl)prop-2-en-1-one 95605-38-2 C9H8O2 148.161 4-甲氧基苯甲醛 4-methoxy-benzaldehyde 123-11-5 C8H8O2 136.15 —— 3-(3,5-Dimethylanilino)-1-(4-methoxyphenyl)propan-1-one —— C18H21NO2 283.37 (2E)-1-(4-甲氧基苯基)-3-苯基-2-丙烯-1-酮 trans-4'-methoxychalcone 22966-19-4 C16H14O2 238.286 —— Dimethyl 2-[3-(4-methoxyphenyl)-3-oxopropyl]propanedioate 1453810-85-9 C15H18O6 294.304 —— [3-(4-Methoxy-phenyl)-3-oxo-propyl]-phosphonic acid diethyl ester 16417-06-4 C14H21O5P 300.292 - 1
- 2
反应信息
-
作为反应物:描述:3-氯-4'-甲氧基苯丙酮 在 sodium azide 、 18-冠醚-6 作用下, 以 二氯甲烷 为溶剂, 生成 3-azido-1-(4-methoxy-phenyl)-propan-1-one参考文献:名称:Synthesis of enantiomerically pure γ-azidoalcohols by lipase-catalyzed transesterification摘要:An enantioselective synthesis of chiral gamma-azidoalcohols via lipase-catalyzed resolution is described. The efficiency of various lipases and the effect of different solvents have been studied. Pseudomonas cepacia immobilized on diatomaceous earth (PS-D) in n-hexane catalyzed the transesterification process in an efficient manner providing gamma-azidoalcohols in high enantiomeric excess. (C) 2008 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetasy.2008.03.028
-
作为产物:描述:参考文献:名称:63.阿朴新烯的组成及其甲基和乙基醚的合成摘要:DOI:10.1039/jr9350000299
文献信息
-
Ultrafast Iron-Catalyzed Reduction of Functionalized Ketones: Highly Enantioselective Synthesis of Halohydrines, Oxaheterocycles, and Aminoalcohols作者:Clemens K. Blasius、Vladislav Vasilenko、Lutz H. GadeDOI:10.1002/anie.201806196日期:2018.8.6A molecularly defined chiral boxmi iron alkyl complex catalyzes the hydroboration of various functionalized ketones and provides the corresponding chiral halohydrines, oxaheterocycles (oxiranes, oxetanes, tetrahydrofurans, and dioxanes) and amino alcohols with excellent enantioselectivities (up to >99 %ee) and conversion efficiencies at low catalyst loadings (as low as 0.5 mol %). Turnover frequencies
-
A convenient enantioselective CBS-reduction of arylketones in flow-microreactor systems作者:Sonia De Angelis、Maddalena De Renzo、Claudia Carlucci、Leonardo Degennaro、Renzo LuisiDOI:10.1039/c6ob00336b日期:——
A convenient, versatile, and green CBS-asymmetric reduction of aryl and heteroaryl ketones has been developed by using the microreactor technology.
一种便捷、多用途且环保的CBS不对称还原芳基和杂芳基酮的方法已经通过使用微反应器技术得以开发。 -
Discovery of 2-(1-(3-(4-Chloroxyphenyl)-3-oxo- propyl)pyrrolidine-3-yl)-1H-benzo[d]imidazole-4-carboxamide: A Potent Poly(ADP-ribose) Polymerase (PARP) Inhibitor for Treatment of Cancer作者:Rui Min、Weibin Wu、Mingzhong Wang、Lin Tang、Dawei Chen、Huan Zhao、Cunlong Zhang、Yuyang JiangDOI:10.3390/molecules24101901日期:——A series of benzimidazole carboxamide derivatives have been synthesized and characterized by 1H-NMR, 13C-NMR and HRMS. PARP inhibition assays and cellular proliferation assays have also been carried out. Compounds 5cj and 5cp exhibited potential anticancer activities with IC50 values of about 4 nM against both PARP-1 and PARP-2, similar to the reference drug veliparib. The two compounds also displayed
-
Enantioselective Cyclizations of Silyloxyenynes Catalyzed by Cationic Metal Phosphine Complexes作者:Jean-François Brazeau、Suyan Zhang、Ignacio Colomer、Britton K. Corkey、F. Dean TosteDOI:10.1021/ja210388g日期:2012.2.8discovery of complementary methods for enantioselective transition metal-catalyzed cyclization with silyloxyenynes has been accomplished using chiral phosphine ligands. Under palladium catalysis, 1,6-silyloxyenynes bearing a terminal alkyne led to the desired five-membered ring with high enantioselectivities (up to 91% ee). As for reactions under cationic gold catalysis, 1,6- and 1,5-silyloxyenynes
-
Efficient AcrH <sub>2</sub> Catalyzed β‐Trifluoromethylation of Carbonyl Compounds by Atom Transfer Radical Addition Reactions作者:Zi‐Peng Rao、Yu‐Yang Sun、Xin‐Feng Zhou、Qiang Xie、Hui‐Xia Zhu、Jian‐Jun Dai、Jun Xu、Hua‐Jian XuDOI:10.1002/cjoc.201900221日期:2019.10The use of organic catalysis AcrH2 enables the direct β‐trifluoromethylation of carbonyl compounds by atom transfer radical addition reactions with broad substrate scopes under mild conditions. This operationally simple and robust protocol successfully converts a variety of β‐chloro carbonyl compounds into the corresponding α‐chloro‐β‐fluoroalkylcarbonyl products. A significant advantage of this method
表征谱图
-
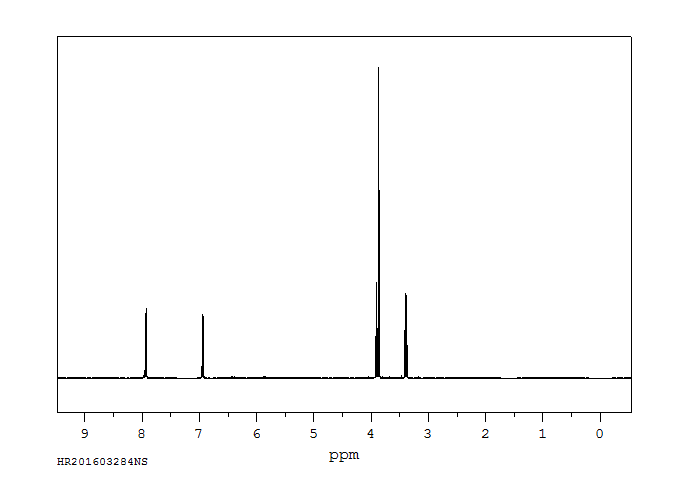
氢谱1HNMR
-
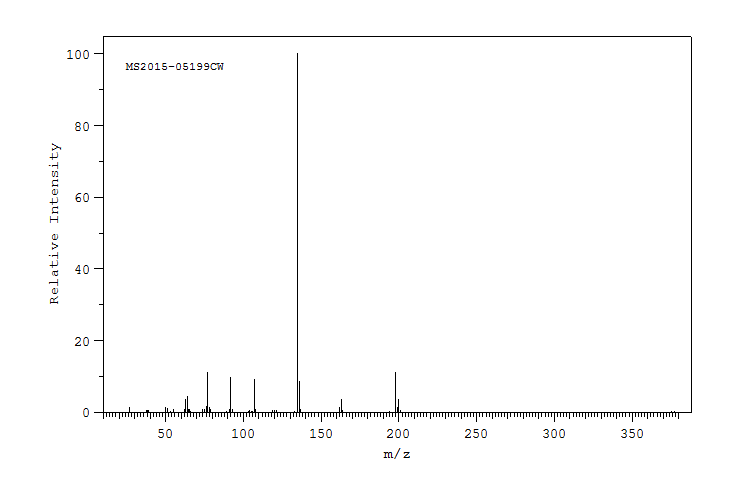
质谱MS
-
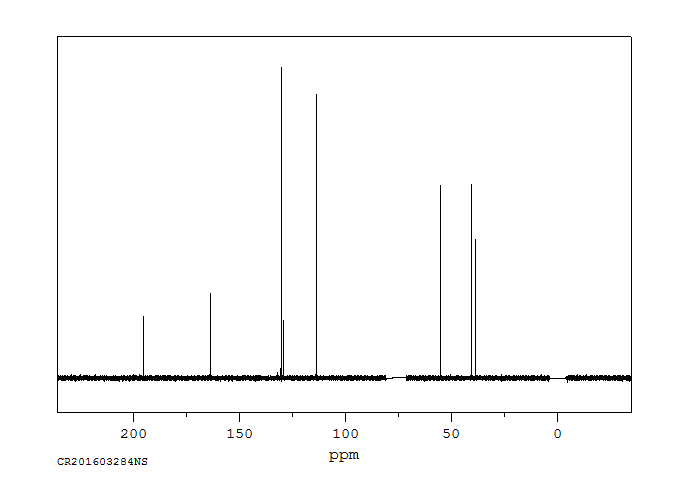
碳谱13CNMR
-
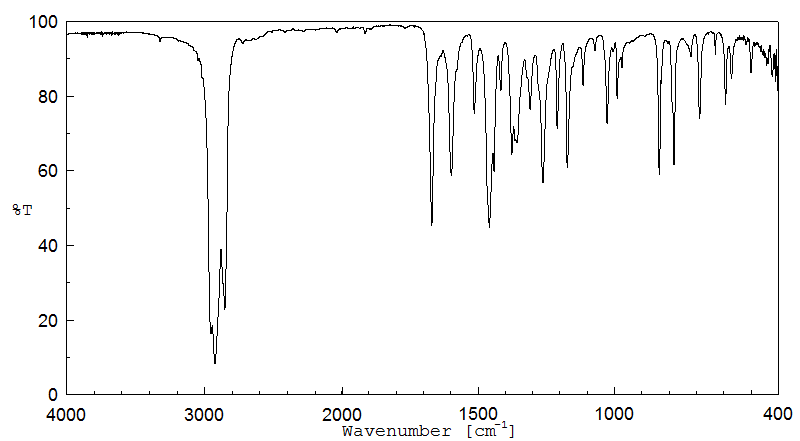
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷