2-甲基-4-羟基苯甲醛 | 41438-18-0
中文名称
2-甲基-4-羟基苯甲醛
中文别名
4-羟基-2-甲基苯甲醛
英文名称
4-hydroxy-2-methyl-benzaldehyde
英文别名
2-methyl-4-hydroxybenzaldehyde;4-Hydroxy-2-methylbenzaldehyde
CAS
41438-18-0
化学式
C8H8O2
mdl
MFCD04037443
分子量
136.15
InChiKey
JDWWIEFMFPWBST-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:104-107°C
-
沸点:205-207 °C(Press: 18 Torr)
-
密度:1.175±0.06 g/cm3(Predicted)
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
危险类别码:R36/37/38
-
海关编码:2912499000
-
安全说明:S26,S36/37/39
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:室温
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
4-Hydroxy-2-methylbenzaldehyde
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
H412: Harmful to aquatic life with long lasting effects
Avoid breathing dust/fume/gas/mist/vapours/spray
P261:
P273: Avoid release to the environment
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
4-Hydroxy-2-methylbenzaldehyde
Ingredient name:
CAS number: 41438-18-0
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H8O2
Molecular weight: 136.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
4-Hydroxy-2-methylbenzaldehyde
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
H412: Harmful to aquatic life with long lasting effects
Avoid breathing dust/fume/gas/mist/vapours/spray
P261:
P273: Avoid release to the environment
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
4-Hydroxy-2-methylbenzaldehyde
Ingredient name:
CAS number: 41438-18-0
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H8O2
Molecular weight: 136.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
2-甲基-4-羟基苯甲醛呈现为白色至淡黄色结晶性粉末,稍溶于水,并可溶于油。
用途该化合物主要用于有机合成,并作为对照品用于复方雪莲胶囊的分析检测。
合成方法将14.4克(0.6摩尔)镁粉加入200毫升甲醇中,开启电动搅拌,瓶内产生大量气泡,温度上升至61℃。两小时后,溶液变为乳白色悬浊液。随后,加入108克(1.0摩尔)对甲基苯酚,在50分钟后开始蒸出甲醇。待反应液变稠时,补充200毫升甲苯,并升高温度直至内温达到95℃。分批加入3.0摩尔的多聚甲醛(90克),改为蒸馏装置以蒸除低沸点物质。约30分钟后几乎无馏分蒸出,撤去蒸馏装置并继续反应2.5小时。使用薄层色谱跟踪,确认对甲基苯酚已完全反应。停止加热,待内温降至65℃时,用18.5%的盐酸调节pH值至1。静置后分液,有机层用70毫升甲苯萃取两次,合并有机层并用水洗三次以调至接近中性pH值。最终得到2-甲基-4-羟基苯甲醛。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-甲氧基-2甲基苯甲醛 4-methoxysalicylaldehyde 52289-54-0 C9H10O2 150.177 3,4-二甲基苯酚 3,4-Dimethylphenol 95-65-8 C8H10O 122.167 4-(甲氧基甲氧基)-2-甲基苯甲醛 2-methyl-4-(methoxymethoxy)benzaldehyde 661481-12-5 C10H12O3 180.203 —— 4-allyloxy-2-methyl-benzaldehyde 206122-92-1 C11H12O2 176.215 4-羟基-2-甲基苯甲腈 4-hydroxy-2-methylbenzonitrile 14143-26-1 C8H7NO 133.15 间甲酚 3-methyl-phenol 108-39-4 C7H8O 108.14 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-甲氧基-2甲基苯甲醛 4-methoxysalicylaldehyde 52289-54-0 C9H10O2 150.177 4-羟基-2-甲基苯甲酸 4-hydroxy-2-methylbenzoic acid 578-39-2 C8H8O3 152.15 —— 2-methyl-4-propoxybenzaldehyde 883531-90-6 C11H14O2 178.231 —— 2-methyl-4-isopropoxybenzaldehyde 86786-25-6 C11H14O2 178.231 —— 4-allyloxy-2-methyl-benzaldehyde 206122-92-1 C11H12O2 176.215 —— 4-(3-chloro-propoxy)-2-methyl-benzaldehyde 848848-22-6 C11H13ClO2 212.676 —— 4-acetoxy-2-methylbenzaldehyde 120802-53-1 C10H10O3 178.188 —— 4-(2-carboxyethoxy)-2-methylbenzaldehyde 661481-13-6 C11H12O4 208.214 —— 4-hydroxymethyl-3-methylphenol 22574-58-9 C8H10O2 138.166 2-甲基-4-苄氧基苯甲醛 4-benzyloxy-2-methyl-benzaldehyde 101093-56-5 C15H14O2 226.275 4-羟基-2-甲基苯甲腈 4-hydroxy-2-methylbenzonitrile 14143-26-1 C8H7NO 133.15 - 1
- 2
反应信息
-
作为反应物:描述:2-甲基-4-羟基苯甲醛 在 哌啶 、 吡啶 、 palladium on activated charcoal 、 乙醇 、 乙酸乙酯 作用下, 生成 3-(4-hydroxy-2-methylphenyl)propionic acid参考文献:名称:Ring Size in the Dienone-Phenol Rearrangement摘要:DOI:10.1021/ja01526a069
-
作为产物:参考文献:名称:取代基对邻甲苯甲醛光化学的影响摘要:研究了4-氧基和4,5-二氧基取代基对2-甲基苯甲醛向邻喹啉二甲烷(o-QDMs)的光化学转化的影响。4-甲氧基或4和5-甲氧基取代基的存在阻止了o-QDM的光化学形成,而4-乙酰氧基和4,5-二乙酰氧基-2-甲基苯甲醛以及相应的甲磺酸酯和甲苯磺酸酯则成功转化为o-QDM。DOI:10.1016/s0040-4039(00)80821-7
文献信息
-
BORON-CONTAINING SMALL MOLECULES申请人:Xia Yi公开号:US20100256092A1公开(公告)日:2010-10-07This invention relates to, among other items, 6-substituted benzoxaborole compounds and their use for treating bacterial infections.这项发明涉及6-取代苯硼酯化合物等物品,以及它们用于治疗细菌感染的用途。
-
[EN] IMIDAZO [2, 1-F] [1, 2, 4] TRIAZIN-4-AMINE DERIVATIVES AS TLR7 AGONIST<br/>[FR] DÉRIVÉS D'IMIDAZO[2,1-F] [1, 2, 4] TRIAZIN-4-AMINE UTILISÉS EN TANT QU'AGONISTES DE TLR7申请人:BEIGENE LTD公开号:WO2020160711A1公开(公告)日:2020-08-13Disclosed herein is an imidazo [2, 1-f] [1, 2, 4] triazin-4-amine derivative or a stereoisomer thereof, or a pharmaceutically acceptable salt thereof useful as a TLR7 agonist, and a pharmaceutical composition comprising the same. Also disclosed herein is a method of treating cancer using the imidazo [2, 1-f] [1, 2, 4] triazin-4-amine derivative or a stereoisomer thereof, or a pharmaceutically acceptable salt thereof as TLR7 agonist.
-
Formylation of Electron-Rich Aromatic Rings Mediated by Dichloromethyl Methyl Ether and TiCl4: Scope and Limitations作者:Iván Ramos-Tomillero、Marta Paradís-Bas、Ibério de Pinho Ribeiro Moreira、Josep Bofill、Ernesto Nicolás、Fernando AlbericioDOI:10.3390/molecules20045409日期:——Here the aromatic formylation mediated by TiCl4 and dichloromethyl methyl ether previously described by our group has been explored for a wide range of aromatic rings, including phenols, methoxy- and methylbenzenes, as an excellent way to produce aromatic aldehydes. Here we determine that the regioselectivity of this process is highly promoted by the coordination between the atoms present in the aromatic moiety and those in the metal core.
-
[EN] BIARYL COMPOUNDS USEFUL AS IMMUNOMODULATORS<br/>[FR] COMPOSÉS BIARYLES UTILES EN TANT QU'IMMUNOMODULATEURS申请人:BRISTOL MYERS SQUIBB CO公开号:WO2018044963A1公开(公告)日:2018-03-08The present disclosure generally relates to compounds useful as immunomodulators. Provided herein are compounds, compositions comprising such compounds, and methods of their use. The disclosure further pertains to pharmaceutical compositions comprising at least one compound according to the disclosure that are useful for the treatment of various diseases, including cancer and infectious diseases.本公开涉及通常用作免疫调节剂的化合物。本文提供了包含这些化合物的组合物,以及它们的使用方法。该公开进一步涉及包含至少一种根据本公开的化合物的药物组合物,用于治疗各种疾病,包括癌症和传染病。
-
Oxazole derivatives申请人:——公开号:US20030055265A1公开(公告)日:2003-03-20The present invention relates to novel oxazole compounds which act as PPAR&agr; and PPAR&ggr; agonists and are accordingly useful for the treatment of diseases modulated by PPAR&agr; and PPAR&ggr; such as diabetes.
表征谱图
-
氢谱1HNMR
-
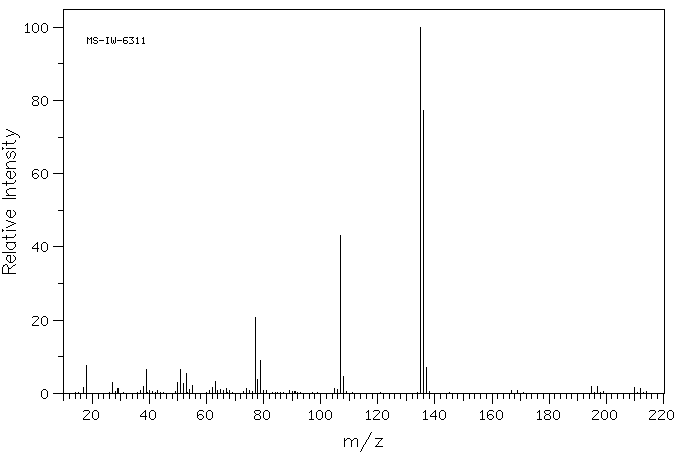
质谱MS
-
碳谱13CNMR
-
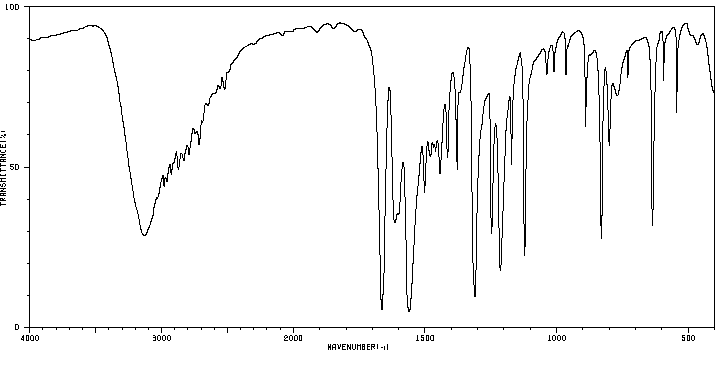
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷