N-丁基乙酰胺 | 1119-49-9
中文名称
N-丁基乙酰胺
中文别名
——
英文名称
N-butylacetamide
英文别名
N-butylethanamide;N-n-butyl acetamide
CAS
1119-49-9
化学式
C6H13NO
mdl
MFCD00041932
分子量
115.175
InChiKey
GYLDXXLJMRTVSS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112-114 °C
-
沸点:117 °C (9 mmHg)
-
密度:0.9
-
保留指数:1090;1057
-
稳定性/保质期:
- 常温常压下稳定,避免与不相容材料接触。
- 可能会与强氧化剂反应。
- 主要存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
海关编码:2924199090
-
WGK Germany:3
-
储存条件:密封保存,应储存在室温和干燥的环境中。
SDS
| Name: | n-Butylacetamide Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1119-49-9 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1119-49-9 | N-butyl acetamide | 100.0 | 214-280-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1119-49-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 117 deg C @ 9.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 115 deg C ( 239.00 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9020g/cm3
Molecular Formula: C6H13NO
Molecular Weight: 115.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1119-49-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N-butyl acetamide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1119-49-9: No information available.
Canada
CAS# 1119-49-9 is listed on Canada's NDSL List.
CAS# 1119-49-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1119-49-9 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-(3-butenyl)acetamide 25420-64-8 C6H11NO 113.159 —— Heptansaeure-butylamid 24928-32-3 C11H23NO 185.31 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(3-氧代丁基)-乙酰胺 N-(3-oxobutyl)acetamide 190452-53-0 C6H11NO2 129.159 —— N-Acetyl-butyramid 22534-71-0 C6H11NO2 129.159 —— N-Butyl-N-methyl-acetamid 10601-67-9 C7H15NO 129.202 —— N-butylpent-4-enamide 107515-69-5 C9H17NO 155.24 —— Butyl-N-fluoroacetamid 23649-63-0 C6H12FNO 133.166 N,N-二丁基乙酰胺 N,N-dibutylacetamide 1563-90-2 C10H21NO 171.283 —— N-butyl-N-chloro-acetamide 502143-93-3 C6H12ClNO 149.62 N-乙基正丁胺 N-ethylbutylamine 13360-63-9 C6H15N 101.192 —— Heptansaeure-butylamid 24928-32-3 C11H23NO 185.31
反应信息
-
作为反应物:参考文献:名称:在温和条件下将酰胺催化加氢为胺摘要:在(不是很大)压力下:已开发出一种使用双金属Pd-Re催化剂将叔酰胺和仲酰胺氢化成胺的通用方法,具有优异的选择性。反应在低压和较低温度下进行。该方法为有机化学家提供了一种简单可靠的胺合成工具。DOI:10.1002/anie.201207803
-
作为产物:描述:参考文献:名称:N-Boc-O-甲苯磺酰基羟胺作为氮源通过贝克曼重排无金属合成仲酰胺摘要:在此,我们报道了第一种使用N -Boc- O-甲苯磺酰羟胺 ( TsONHBoc ) 作为胺化剂通过贝克曼重排从酮合成仲酰胺的直接方法。该试剂有望发挥双重作用,首先是形成活化的肟中间体,然后通过甲苯磺酸副产物促进酰胺形成为布朗斯台德酸。无金属和无添加剂一步法在 TFE 溶剂中通过原位生成伯胺试剂 (TsONH 2 ) 进行,并产生水溶性副产物。DOI:10.1039/d2nj02755k
-
作为试剂:描述:参考文献:名称:Decomposition into radicals and chain decomposition of N-butyl- and ethylacetamide hydroperoxides in n-butylacetamide medium摘要:DOI:10.1007/bf00950631
文献信息
-
RENIN INHIBITORS申请人:Jones Benjamin公开号:US20100210635A1公开(公告)日:2010-08-19Compounds, pharmaceutical compositions, kits and methods are provided for use with Renin that comprise a compound selected from the group consisting of: wherein the variables are as defined herein.提供了用于与Renin一起使用的化合物、药物组合物、试剂盒和方法,其中包括从以下组中选择的化合物:其中变量如本文所定义。
-
The Selective<i>N</i>-Monoalkylation of Amides with Alkyl Halides in the Presence of Alumina and KOH作者:Kazuaki SukataDOI:10.1246/bcsj.58.838日期:1985.3The effects of alumina impregnated with KOH (KOH/Al2O3) and a mixture of alumina and powdered KOH (KOH+Al2O3) have been examined using the alkylation of benzamide under various conditions. In each case, alkylation occurs exclusively in the pores of the alumina, the extent depending upon the alumina-pore size. For both yield and selectivity for N-monoalkylation, KOH+Al2O3 is superior to KOH/Al2O3. Dioxane is the best of the solvents employed. It is proposed that, in dioxane, an enolate-like species, (Remark: Graphics omitted.), exists as stable ion-pair aggregates, which are the true reactants in the pore. This method, using KOH/Al2O3 or KOH+Al2O3, has been applied to N-alkylation of the other amides, giving the N-monoalkylated products in substantial yields with extremely high selectivities. 2-Phenylacetamide is regioselectively N-monoalkylated in high yields. This regioselectivity is explained in terms of steric hindrance.使用KOH浸渍的氧化铝(KOH/Al2O3)和氧化铝与粉末状KOH的混合物(KOH+Al2O3),在不同条件下研究了苯甲酰胺的烷基化反应。在每种情况下,烷基化反应仅在氧化铝的孔隙中发生,其程度取决于氧化铝的孔径大小。对于N-单烷基化的产率和选择性,KOH+Al2O3优于KOH/Al2O3。二氧六环是所使用溶剂中最好的。研究表明,在二氧六环中,类似于烯醇盐的物种(注:图形省略)以稳定的离子对聚集形式存在,这些是孔隙中的真正反应物。这种方法使用KOH/Al2O3或KOH+Al2O3,已应用于其他酰胺的N-烷基化,以极高的选择性获得了大量的N-单烷基化产物。2-苯基乙酰胺以高产率选择性地进行N-单烷基化。这种区域选择性可通过空间位阻来解释。
-
A CO<sub>2</sub>-Catalyzed Transamidation Reaction作者:Yang Yang、Jian Liu、Fadhil S. Kamounah、Gianluca Ciancaleoni、Ji-Woong LeeDOI:10.1021/acs.joc.1c02077日期:2021.12.3Transamidation reactions are often mediated by reactive substrates in the presence of overstoichiometric activating reagents and/or transition metal catalysts. Here we report the use of CO2 as a traceless catalyst: in the presence of catalytic amounts of CO2, transamidation reactions were accelerated with primary, secondary, and tertiary amide donors. Various amine nucleophiles including amino acid在过量化学计量的活化试剂和/或过渡金属催化剂的存在下,转酰胺基反应通常由反应性底物介导。在这里,我们报告了使用 CO 2作为无痕催化剂:在催化量的 CO 2存在下,使用伯、仲和叔酰胺供体加速转酰胺反应。包括氨基酸衍生物在内的各种胺类亲核试剂都被耐受,这表明转酰胺基在肽修饰和聚合物降解中的实用性(例如,Nylon-6,6)。特别是,N , O -二甲基羟基酰胺(Weinreb 酰胺)在 CO 2催化的转酰胺基反应中与 N 2相比表现出明显的反应性大气层。进行了比较 Hammett 研究和动力学分析,以阐明分子 CO 2的催化活化机制,这得到了 DFT 计算的支持。我们将CO 2在转酰胺基反应中的积极作用归因于通过与亲电子CO 2共价结合来稳定四面体中间体。
-
Dehydrogenative Coupling of Ethanol and Ester Hydrogenation Catalyzed by Pincer-Type YNP Complexes作者:Dmitry G. GusevDOI:10.1021/acscatal.6b02324日期:2016.10.7The “Y” donor group (Y = −OMe, −SEt, −PPh2, −NH2, −NMe2, −Py, pyrrolidinyl, quinolyl) of the pincer-type ruthenium complexes RuHCl(CO)[κ3-YNP] has a dramatic influence on the catalytic activity in the dehydrogenative homocoupling and cross-coupling of ethanol and ester hydrogenation reactions. The observations are connected with the mechanisms of the catalytic reactions, and this paper provides evidence
-
Synthesis and Application of a Microgel-Supported Acylating Reagent by Coupled Ring-Opening Metathesis Polymerization and Activators Re-Generated by Electron Transfer for Atom Transfer Radical Polymerization作者:Hong Li、Zi-Bo Pang、Zhi-Feng Jiao、Fei LinDOI:10.1021/cc900162w日期:2010.3.8microgel-supported acylating reagent (MGAR) was prepared by combining ring-opening metathesis polymerization (ROMP) and Activators Re-Generated by Electron Transfer for Atom Transfer Radical Polymerization (ARGET ATRP): (1) synthesis of an ATRP macroinitiator 3 by living ROMP of oxanorbornene-based activated ester 1, derived from N-hydroxysuccinimide, using the Grubbs initiator RuCl2(PCy3)2(=CHPh) and (Z)-but-2-ene-1一种新的微凝胶支持的酰化试剂(MGAR)的制备是通过组合开环易位聚合(ROMP),并通过电子转移进行原子转移自由基聚合(ARGET ATRP)活化剂重新生成:(1)一个ATRP大分子引发剂的合成3通过使用Grubbs引发剂RuCl 2(PCy 3)2(= CHPh)和(Z)-but-2-ene-1,4-diyl bis(2)从N-羟基琥珀酰亚胺衍生的基于氧杂降冰片烯的活化酯1的活性ROMP -溴丙酸酯(BDBP)作为终止剂;(2)使用制备的大分子引发剂3,CuCl 2通过ARGET ATRP苯乙烯(S)和二乙烯基苯(DVB )合成MGAR 4。/ Me 6 TREN(三[2-(二甲基氨基)乙基]胺)催化剂体系,Sn(Oct)2 [锡(II)2-乙基己酸]还原剂。合成的微凝胶4在温和条件下(25°C,13.5-14 h)对伯胺和仲胺(n- BuNH 2,Et 2 NH,吗啉等)表现出优异的酰
表征谱图
-
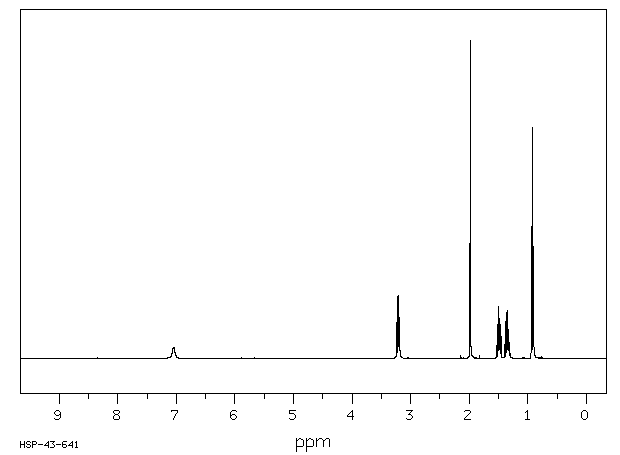
氢谱1HNMR
-
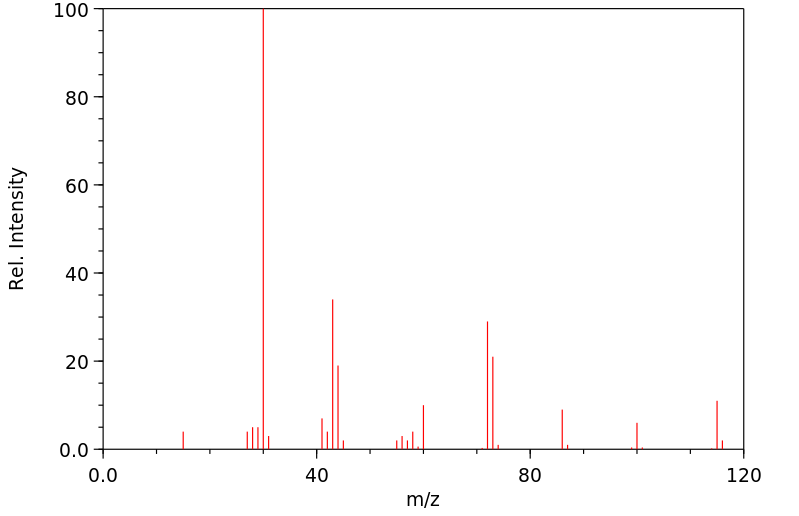
质谱MS
-
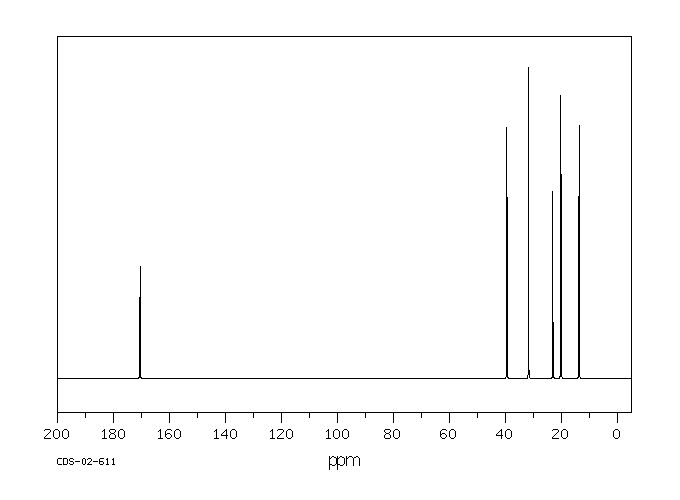
碳谱13CNMR
-
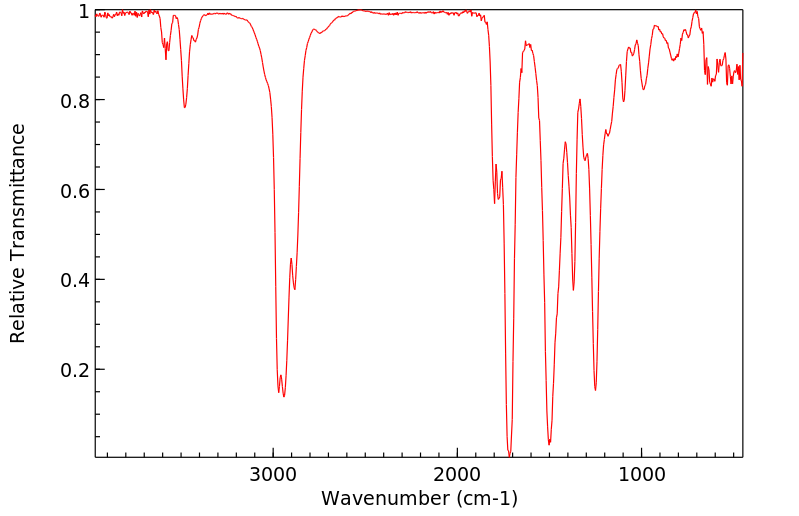
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸